Doubt by rakesh narabherambhai baraiya
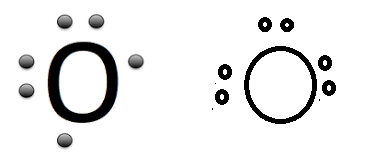
maam yesterday when i asked you the doubt about the correct lewis dot structure of oxygen atom then you said that the first image is correct BUT in NCERT second image is given....
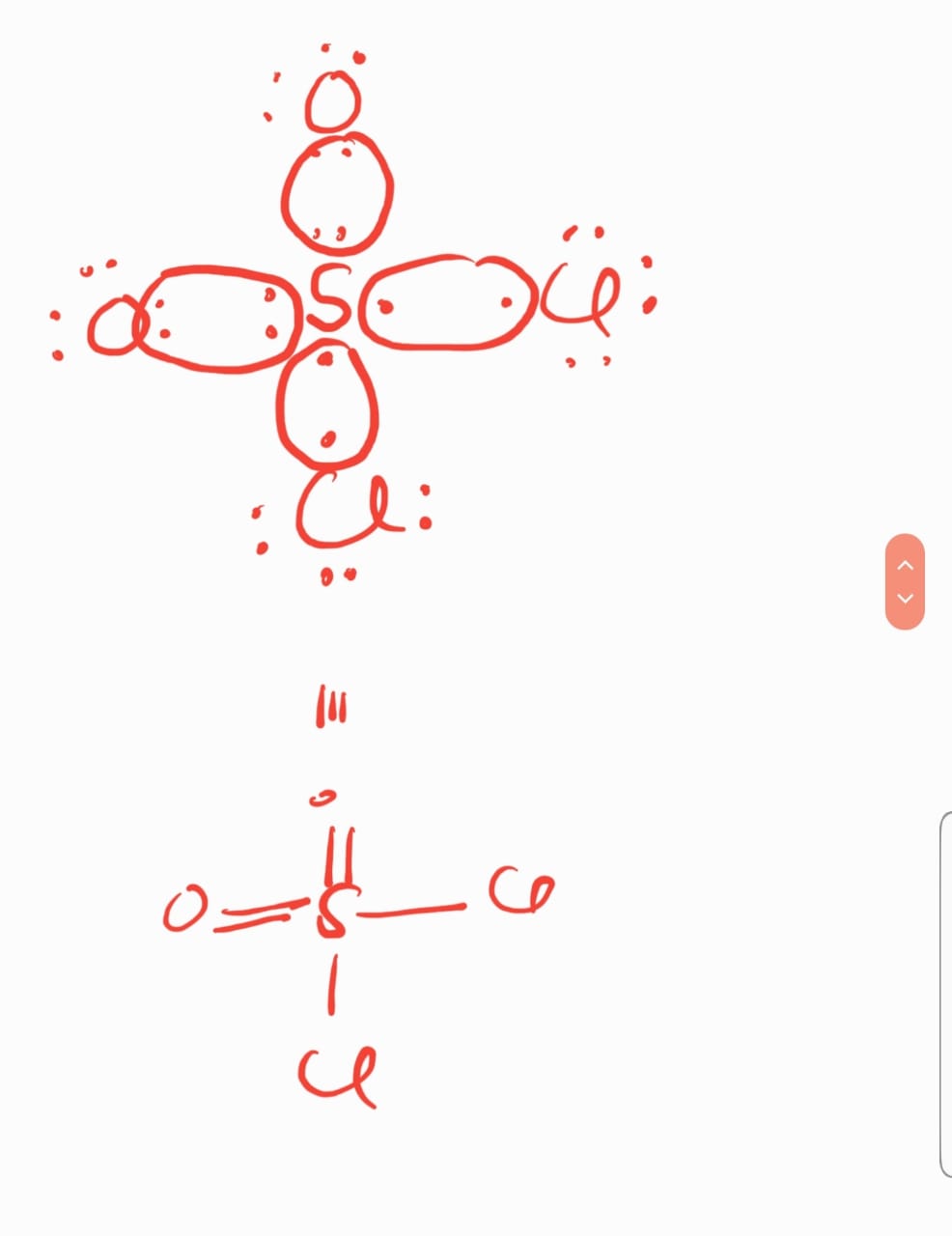
AND when we draw structure of S02Cl2 using lewis dot structure of ncert then there are two dative bond But If we use lewis dot structure given in 2nd image then there are two covalent bonds.. why is there difference?
please solve this because i am much confused?
Answers
Answer by rakesh narabherambhai baraiya
sorrry maam in second last sentence it is 1st image
Answer by Dr. Rakhee
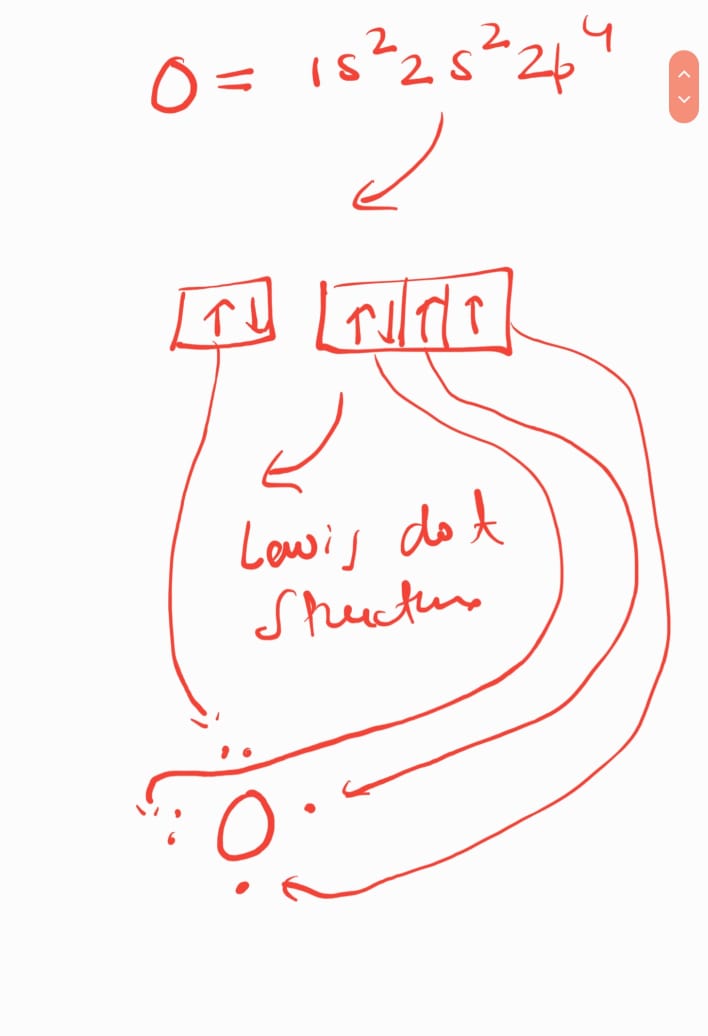
DEAR THE FIRST STRUCTURE IS CORRECT AND I SEND YOU THE EXPLANATION...
AND I ALSO SEND YOU THE LEWIS DOT STRUCTURE OF SO2CL2
KINDLY CHECK AND LET ME KNOW IF YOU FEEL ANY PROBLEM....