Answers
Answer by Dr. Rakhee
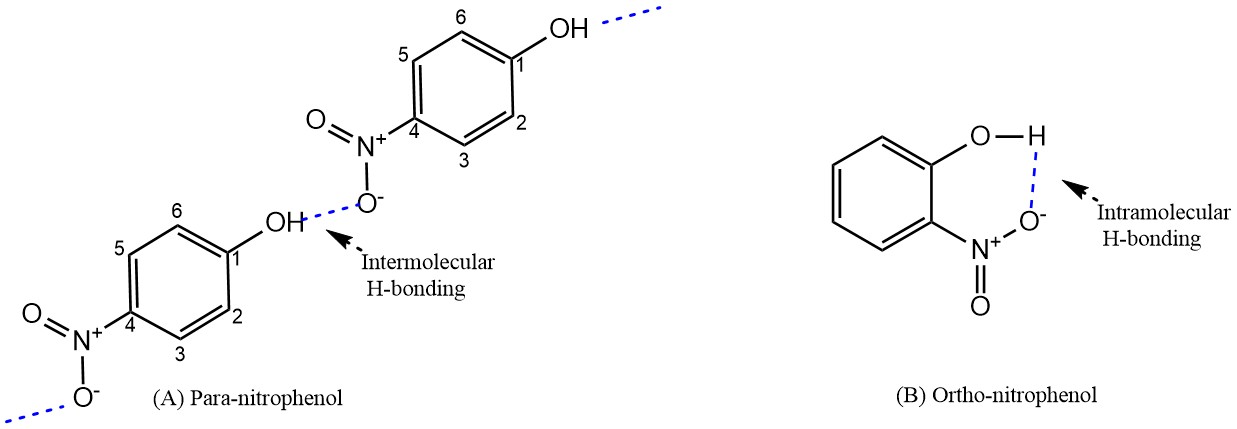
(a) Higher than that of A
Reason: P-nitrophenol form Intermolecular H-bonding whereas O-nitrophenol form Intramolecular H-bonding. And O-nitrophenol is more volatile in nature. Because during boiling, the strong intermolecular H-bonding increases the boiling point but intramolecular H- bonding can't do it.

- 8527521718
- support@neetprep.com
- S-15, 2nd floor Uphar Cinema Market, above Red Chilli Restaurant, Green Park Extension, New Delhi, 110016
Botany Questions
- Living World
- Biological Classification
- Plant Kingdom
- Morphology of Flowering Plants
- Anatomy of Flowering Plants
- Cell-unit of Life
- Cell Cycle and Cell Division
- Transport in Plants
- Mineral Nutrition
- Photosynthesis in Higher Plants
- Respiration in Plants
- Plant Growth and Development
- Reproduction in Organisms
- Sexual Reproduction in Flowering Plants
- Principles of Inheritance and Variation
- Molecular Basis of Inheritance
- Strategies for Enhancement in Food Production
- Microbes in Human Welfare
- Organisms and Populations
- Ecosystem
- Biodiversity and Conservation
- Environmental Issues
Chemistry Questions
- Some Basic Concepts of Chemistry
- Structure of Atom
- Classification of Elements and Periodicity in Properties
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The s-Block Elements
- The p-Block Elements-XI
- Organic Chemistry - Some Basic Principles and Techniques
- Hydrocarbons
- Environmental Chemistry
- The Solid State
- Solutions
- Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- General Principles and Processes of Isolation of Elements
- The p-Block Elements-XII
- The d and f Block Elements
- Coordination Compounds
- Haloalkanes and Haloarenes
- Alcohols, Phenols and Ethers
- Aldehydes, Ketones and Carboxylic Acids
- Amines
- Biomolecules
- Polymers
- Chemistry in Everyday Life
Physics Questions
- Units and Measurement
- Mathematical Tools
- Motion in A Straight Line
- Motion in A Plane
- Laws of Motion
- Work, Energy and Power
- Systems of Particles and Rotational Motion
- Gravitation
- Mechanical Properties of Solids
- Mechanical Properties of Fluids
- Thermal Properties of Matter
- Thermodynamics
- Kinetic Theory of Gases
- Oscillations
- Waves
- Electric Charges and Fields
- Electrostatic Potential and Capacitance
- Current Electricity
- Moving Charges and Magnetism
- Magnetism and Matter
- Electromagnetic Induction
- Alternating Current
- Electromagnetic Waves
- Ray Optics and Optical Instruments
- Wave Optics
- Dual Nature of Radiation and Matter
- Atoms
- Nuclei
- Semiconductor Electronics
Zoology Questions
- Animal Kingdom
- Structural Organisation in Animals
- Biomolecules
- Digestion and Absorption
- Breathing and Exchange of Gases
- Body Fluids and Circulation
- Excretory Products and their Elimination
- Locomotion and Movement
- Neural Control and Coordination
- Chemical Coordination and Integration
- Human Reproduction
- Reproductive Health
- Evolution
- Human Health and Disease
- Biotechnology Principles and Processes
- Biotechnology and its Application
© 2026 GoodEd Technologies Pvt. Ltd.