Which of the following will give yellow precipitate with I2/NaOH?
| 1. | CH3-CO-O-CO-CH3 | 2. |  |
| 3. |  |
4. | Both (2) and (3) |
Identify (X) in the sequence :
1. CH3-CH2-CH2OH
2.
3. CH3-O-CH2-CH3
4. CH3-CH2-CHO
An organic compound X on treatment with acidified K2Cr2O7, gives compound Y which reacts with I2, and NaOH to form CHI3. The compound X can be-
1. CH3OH
2. CH3CHO
3. CH3COCH3
4. CH3CH(OH)CH3
Which compound would give 5-keto-2-methyl hexanal upon ozonolysis?
| 1. | 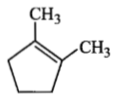 |
2. | 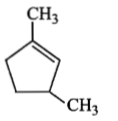 |
| 3. | 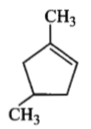 |
4. | 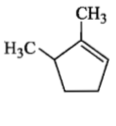 |
CH3CH=CHCHO is oxidized to CH3CH=CHCOOH using:
1. Alkaline permanganate
2. Ammoniacal silver nitrate
3. Selenium dioxide
4. Osmium tetroxide
Ozonolysis of propyne gives:
1. CH3CHO
2. CH3COCHO
3. HCHO
4. CHOCHO
Propyne on oxidation with SeO2 gives:
1. CHOCHO
2. CH3CH2CHO
3. CH3COCHO
4. CHOCH2CHO
Acetylene and HCHO react in the presence of copper acetylide catalyst to form:
(1) 2-Butyne-1,4-diol
(2) 1-Butyne-1,4-diol
(3) 2-Butyne-1,2-diol
(4) None of these
An organic compound X having molecular formula C5H10O yields phenyl hydrazone and
gives negative response to the iodoform test and Tollen's test. It produces n-pentane on
reduction. X could be:
1. Pentenal
2. 2-Pentanone
3. 3-Pentanone
4. n-Amyl alcohol
CH3CHO and C6H5CH2CHO can be distinguished chemically by:
1. Benedict test
2. Iodoform test
3. Tollen's reagent test
4. Fehling solution test






