A system is taken from state \(A\) to state \(B\) along two different paths \(1\) and \(2.\) If the heat absorbed and work done by the system along these two paths are \(Q_1,Q_2\) and \(W_1,W_2\) respectively, then
1. \(Q_1=Q_2\)
2. \(W_1=W_2\)
3. \(Q_1-W_1=Q_2-W_2\)
4. \(Q_1+W_1=Q_2+W_2\)
In a given process, dW = 0, dQ < 0, then for the gas:
1. Temperature increases
2. Volume decreases
3. Pressure decreases
4. Pressure increases
An ideal gas with adiabatic exponent y is heated at constant pressure and it absorbs Q heat. What fraction of this heat is used to perform external work?
1.
2.
3.
4.
Figure below shows two paths that may be taken by a gas to go from a state \(A\) to a state \(C.\) In process \(AB,\) \(400\text{ J}\) of heat is added to the system and in process \(BC,\) \(100\text{ J}\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be-
1. \(380\text{ J}\)
2. \(500\text{ J}\)
3. \(460\text{ J}\)
4. \(300\text{ J}\)
A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
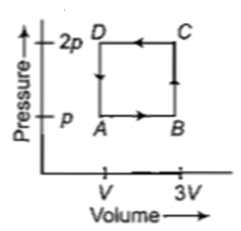
1. 2 pV
2. 4 pV
3.
4. pV
An ideal gas goes from state \(A\) to state \(B\) via three different processes, as indicated in the \(P\text-V\) diagram. If \(Q_1,Q_2,Q_3\) indicates the heat absorbed by the gas along the three processes and \(\Delta U_1, \Delta U_2, \Delta U_3\) indicates the change in internal energy along the three processes respectively, then:

| 1. | \({Q}_1>{Q}_2>{Q}_3 \) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 2. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 3. | \({Q}_1={Q}_2={Q}_3\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
| 4. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
The internal energy change in a system that has absorbed 2 kcal of heat and done 500 J of work is
1. 8900 J
2. 6400 J
3. 5400 J
4. 7900 J
A system performs work ΔW when an amount of heat is ΔQ added to the system, the corresponding change in the internal energy is ΔU. A unique function of the initial and final states (irrespective of the mode of change) is -
(1) ΔQ
(2) ΔW
(3) ΔU and ΔQ
(4) ΔU
A container of volume 1m3 is divided into two equal compartments by a partition. One of these compartments contains an ideal gas at 300 K. The other compartment is vaccum. The whole system is thermally isolated from its surroundings. The partition is removed and the gas expands to occupy the whole volume of the container. Its temperature now would be -
(1) 300 K
(2) 239 K
(3) 200 K
(4) 100 K
110 J of heat is added to a gaseous system, whose internal energy change is 40 J, then the amount of external work done is
(1) 150 J
(2) 70 J
(3) 110 J
(4) 40 J







