Acetamide and ethyl amine can be distinguished by reacting with:
1. Aq. HCl and heat
2. Aq. NaOH and heat
3. Acidified KMnO4
4. Bromine water
Predict the product,

| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Intermediates formed during the reaction of RCONH2 with Br2 and KOH are -
1. RCONHBr and RNCO
2. RNHCOBr and RNCO
3. RNHBr and RCONHBr
4. RCONBr2
Aniline in a set of the following reactions yielded a coloured product Y.
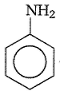
The structure of Y would be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Name the end product in the following series of reactions,
CH3COOH ABC:
1. CH4
2. CH3OH
3. acetonitrile
4. ammonium acetate
 and
and ![]()
What type of isomers are they?
1. Chain
2. Functional
3. Position
4. All of the above
The numbers of primary amines posible for the formula C4H11N is -
1. 1
2. 2
3. 3
4. 4
The type of isomerism shown by C6H5CN and C6H5NC is:
1. Position
2. Functional
3. enantiomer
4. Tautomerism
The product D in the following sequence of reactoins is,
:
1. Ester
2. Amine
3. Acid
4. Alcohol
The IUPAC name of the compound having formula,
is :
1. 3-Aminohydroxypropionic acid
2. 2-Amino-propan-3-oic acid
3. Amino hydroxy propionic acid
4. 2-Amino-3-hydroxy propanoic acid






