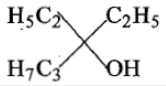
In the following reaction,
an asymmetric center is generated. The acid obtained would be
1. L-isomer
2. D-isomer
3. 20% D+ 80% L-isomer
4. 50% D+ 50 % L-isomer
The major organic product formed from the following reaction


| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1.
2.
3.
4.
A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of -hydroxy acid. The carbonyl compound is
1. acetaldehyde
2. acetone
3. diethyl ketone
4. formaldehyde
A and B in the following reactions are 


1. A=, B=NaOH
2. A=
3. A=
4. A=
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Consider the following reaction,
PhenolXYZ
The product Z is
1. toluene
2. benzaldehyde
3. benzoic acid
4. benzene
The compound formed when malonic acid heated with urea, is
1. cinnamic acid
2. butyric acid
3. barbituric acid
4. crotonic acid
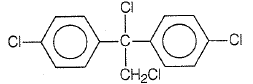
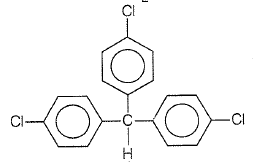
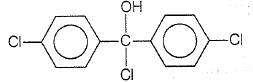
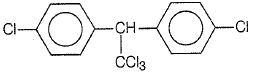
Trichloroacetaldehyde, CCl3CHO reacts with chlorobenzene in the presence of sulphuric acid and produces
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1. 
2. 
3. 
4. 
What compound results from the catalytic dehydrogenation of a primary alcohol?
1. Aldehyde
2. Ketone
3. Alkene
4. Acid
The compound which on reduction with gives two diffrent alcohols:
1.
2.
3.
4.
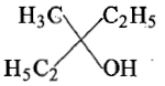
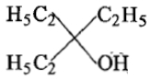
Ethyl ester P. the product P will be:
1. 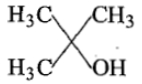

2. 

3. 

4.