The major product of the following reaction sequence will be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Select the correct option based on statements below:
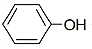
| Assertion (A): | -OH, -OCH3 groups are o, p-directing. |
| Reason (R): | Ring is activated by the resonance effect of -OH, -OCH3 groups. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Select the correct option based on statements below:
| Assertion (A): | Like the bromination of benzene, bromination of phenol is carried out in the presence of Lewis acid. |
| Reason (R): | Lewis acid polarizes the bromine molecule. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Select the correct option based on the statements below:
| Assertion (A): | Phenols give o-and p-nitrophenol on nitration with conc. HNO3 and H2SO4 mixture. |
| Reason (R): | —OH group in phenol is o-, p-directing. |
| 1. | Both (A) and (R) are True, and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
The group that activates the benzene ring towards electrophilic substitution reaction is:
| 1. | OH | 2. | NO2 |
| 3. | CN | 4. | COOH |
The alkoxy group in aryl alkyl ethers activates the benzene ring towards:
1. Nucleophilic addition reaction
2. Electrophilic addition reaction
3. Nucleophilic substitution reaction
4. Electrophilic substitution reaction
An appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene is/are :
| 1. |  |
| 2. |  |
| 3. | \(\mathrm{CH_3-CH_2-CH_2-O-CH_3+HBr}\) |
| 4. | \(\mathrm{(CH_3)_3C-OC_2H_5+HI}\) |
In Kolbe’s reaction,

the product formed will be:
1. para-Salicylaldehyde
2. ortho-Salicylaldehyde
3. para-Salicylic acid
4. ortho-Salicylic acid
In Reimer-Tiemann reaction,

the product will be:
1. ortho-Salicylaldehyde
2. para-Salicylaldehyde
3. meta-Salicylaldehyde
4. All of the above
In the following Williamson ether synthesis reaction, what will be the major product?

1. 1-Methoxypropane
2. 2-Methoxypropane
3. 3-Methoxypropane
4. All of the above






