Compound (A), C8H9Br , gives a white precipitate when warmed with alcholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
| 1. |   |
| 2. |   |
| 3. |   |
| 4. |   |
Nitrobenzene on electrolytic reduction in a strongly acidic medium gives:
1. Aniline
2. p-Aminophenol
3. m-Nitroaniline
4. Nitrosobenzene

(1) CF3CO3H
(2) H2SO4
(3) LAH
(4) NaBH4

can be differentiated by:
1. Hinsberg test
2. Iso-cyanide test
3. NaNO2, HCl, then β-Naphthol
4. NaOH


(p-ethyl phenol) (p-methyl anisole) (p-ethyl benzyl alcohol)
Above compounds can be differentiated by using the reagent:-
(a) NaOH, Tollen's reagent, FeCl3
(b) CrO3, Tollen's reagent, FeCl3,
(c) Tollen's reagent, CrO3, FeCl3
(d) Na, Tollen's reagent, FeCl3
Give test to differentiate (Bromobenzene) Ph-Br and benzyl bromide (PhCH2Br).
(1) (i) aq. KOH (ii) Na
(2) AgNO3
(3) KMnO4
(4) All these
In a reaction,
A is
1. HgSO4/H2SO4
2. Cu2Cl2
3. H3PO2 and H2O
4. H+/H2O
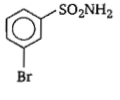
In a set of reactions, m-bromobenzoic acid gave a product D. Identify the product D.

1.
2.
3.
4. 
What is the product obtained in the following reaction?

 , Product (C) is:
, Product (C) is:












