An organic compound (A) on reduction gives compound (B) which on reaction with chloroform and potassium hydroxide forms (C). The compound (C) on catalytic reduction gives N-methyl aniline. What is the structure of compound (A)?
1. Nitrobenzene
2. Nitromethane
3. Methylamine
4. Aniline
 and
and ![]()
What type of isomers are they?
1. Chain
2. Functional
3. Position
4. All of the above
Primary, secondary and tertiary nitroalkanes can be identifier by the action of:
1.
2.
3.
4. none of these
A nitrogenous substance X is treated with HNO2 and the product so formed is further treated with NaOH solution, which produces blue colouration. X can be:
1.
2.
3.
4.
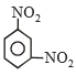

Product (C) is
1. Nitrobenzene
2. 1,3-Diethoxybenzenez
3. Ethoxybenzene
4. Benzene
Which of the following is regenerated at the end of the reaction
(1) X
(2) Y
(3) Z
(4) W
Product (C) in the above reaction is:
1.
2.
3.
4.
upon heating generates -
1. Nitrogen gas
2. Carbon dioxide
3. Biuret
4. Ammonium carbonate
An organic compound X (mol. formula C6H5O2N) has 6 carbon atoms in a ring system, three double bonds and also a nitro group as substituent X is:
1. Homocyclic but not aromatic
2. Aromatic but not homocyclic
3. Homocyclic and aromatic
4. Heterocyclic
A given nitrogen-containing aromatic compound a reacts with Sn/HCl, followed by HNO2 to give an unsatable compound B.B, on treament with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is













