The increasing order of hydrolysis of the following compounds:

1. (i)<(iii)<(ii)
2. (ii)<(iii)<(i)
3. (ii)<(i)<(iii)
4. (i)<(ii)<(iii)
What is major product of following reaction ?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Consider the given reaction below:
Which of these is true regarding the reaction shown above?
| 1. | The configuration of the chiral carbon remains the same. |
| 2. | The configuration of the chiral carbon gets inverted |
| 3. | The compound formed as a product must be a dextro isomer |
| 4. | The reactant is optically inactive but the product is obtained as a levo isomer. |
The ease of dehydrohalogen of alkyl halide with alcoholic KOH is
1. 3º > 2º > 1º
2. 3º < 2º < 1º
3. 3º > 2º < 1º
4. 3º < 2º > 1º
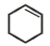
Which of the following is formed by the thermal decomposition of the hydroxide of:
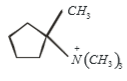
1. 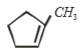
2. 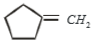
3. 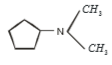
4. 
Read the following road map carefully
(1) Both the ethers obtained by the two routes have opposite but equal optical rotation.
(2) One of the ether is obtained as a racemic mixture.
(3) Step II & III both are SN2 reaction and both have inversion.
(4) Step II has inversion but step III has retention.
Which of the following statement is correct
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |





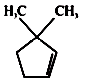
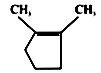



 A B
A B





