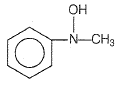
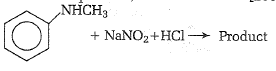An organic compound (A) on reduction gives compound (B) which on reaction with chloroform and potassium hydroxide forms (C). The compound (C) on catalytic reduction gives N-methyl aniline. What is the structure of compound (A)?
1. Nitrobenzene
2. Nitromethane
3. Methylamine
4. Aniline
Acetamide and ethyl amine can be distinguished by reacting with:
1. Aq. HCl and heat
2. Aq. NaOH and heat
3. Acidified KMnO4
4. Bromine water
Which of the following compound gives dye test
1. Aniline
2. Methylamine
3. Diphenylamine
4. Ethylamine
CH3CH2ClXYZ
In the above reaction sequence, Z is
1. CH3CH2CH2NHCOCH3
2. CH3CH2CH2NH2
3. CH3CH2CH2CONHCH3
4. CH3CH2CH2CONHCOCH3
Aniline in a set of reactions yield the following products


The structure of the product D would be -
1. C6H5CH2NH2
2. C6H5NHCH2CH3
3. C6H5NHOH
4. C6H5CH2OH
Intermediates formed during the reaction of RCONH2 with Br2 and KOH are -
1. RCONHBr and RNCO
2. RNHCOBr and RNCO
3. RNHBr and RCONHBr
4. RCONBr2
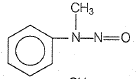
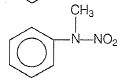
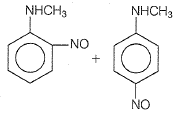
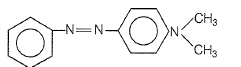
In a reaction of aniline a colored product C was obtained.
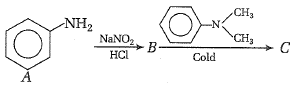

The structure of C would be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1. 
2. 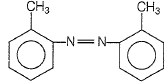
3. 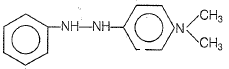
4. 


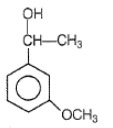
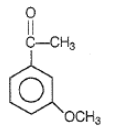
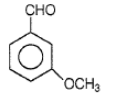
Product Q in the above reaction is
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1. 
2. 
3. 
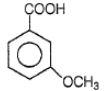
4. 
The final product C in the below mentioned reaction is:

 \(\xrightarrow[]{Ac_2O}\ A\ \xrightarrow[CH_3COOH]{Br_2}\ B\ \xrightarrow[H^+]{H_2O}\ C\)
\(\xrightarrow[]{Ac_2O}\ A\ \xrightarrow[CH_3COOH]{Br_2}\ B\ \xrightarrow[H^+]{H_2O}\ C\)
| 1. |  |
2. |  |
| 3. |  |
4. |  |