| 1. |  |
2. |  |
| 3. |  |
4. |  |
The major product formed when cyclohexanecarbaldehyde reacts with PhMgBr and H3O+ is:
| 1. |  |
2. |  |
| 3. |  |
4. | None of these |
An alkene “A” on reaction with O3 and Zn - H2O gives propanone and ethanal in an equimolar ratio. The addition of HCl to alkene “A” gives “B” as the major product. The structure of product “B” is:
| 1. | 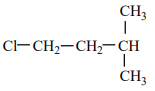 |
2. | 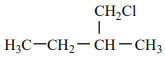 |
| 3. | 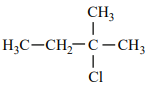 |
4. | 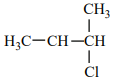 |
A compound A, when reacted with PCl5 and then with ammonia, gave B. B, when treated with bromine and caustic potash, produced C. C on treatment with NaNO2 and HCl at C and on boiling produced ortho-cresol. Compound A is:
| 1. | o-Toluic acid | 2. | o-Chlorotoluene |
| 3. | o-Bromotoluene | 4. | m-Toluic acid |
Identify product 'B' in the given reaction:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
The formation of cyanohydrin from acetone is an example of:
1. Nucleophilic substitution.
2. Electrophilic substitution.
3. Electrophilic addition.
4. Nucleophilic addition.
The below structure is an example of :

| 1. | Cyanohydrin | 2. | Hemiacetal |
| 3. | Acetal | 4. | Cyanoalcohol |
The nucleophilic addition reaction will be most favored among the given compounds is:
1.
2.
3.
4.
CH3CHO and C6H5CH2CHO can be distinguished by:
1. Benedict test
2. Iodoform test
3. Tollen's reagent test
4. Fehling solution test


| (i) | (ii) | (iii) | |
| 1. | CH3 – MgI, H3O+ | H2SO4, ∆ | HBr, R2O2 |
| 2. | CH3 – MgI, H3O+ | H2SO4, ∆ | HBr |
| 3. | CH3 – MgI, H3O+ | HBr | |
| 4. | HBr, R2O2 | CH3 – MgI, H3O+ |




