The hydrogenation of benzoyl chloride in the presence of Pd and BaSO4 gives:
1. Benzyl alcohol
2. Benzaldehyde
3. Benzoic acid
4. Phenol
An alkene “A” on reaction with O3 and Zn - H2O gives propanone and ethanal in an equimolar ratio. The addition of HCl to alkene “A” gives “B” as the major product. The structure of product “B” is:
| 1. | 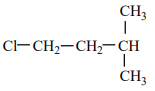 |
2. | 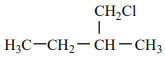 |
| 3. | 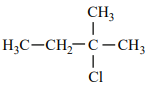 |
4. | 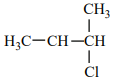 |
Identify product 'B' in the given reaction:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
The formation of cyanohydrin from acetone is an example of:
1. Nucleophilic substitution.
2. Electrophilic substitution.
3. Electrophilic addition.
4. Nucleophilic addition.
2-Methyl propene on oxidation with hot KMnO4 gives:
1. Acetone
2. Ethanoic acid
3. CO2 and H2O
4. Both 1 & 3
What is the most suitable reagent for the below mentioned conversion?
\(\mathrm{CH_{3} — CH = CH — CH_{2} — \overset{\Large{O} \\~ ||}{C}—CH_{3} \rightarrow}\)
\(\mathrm{CH_{3} — CH = CH — CH_{2} — \overset{\Large{O} \\~ ||}{C}—OH}\)
1. Tollens' reagent
2. Benzoyl peroxide
3. \(\mathrm I_{2}\) and NaOH solution
4. Sn and NaOH solution
Identify the conversions that are possible with Clemmensen reduction:
a. Benzaldehyde to benzyl alcohol.
b. Cyclohexanone to cyclohexane.
c. Benzoyl chloride to benzaldehyde.
d. Benzophenone to diphenylmethane.
| 1. | a and b | 2. | b and c |
| 3. | c and d | 4. | b and d |
Match the common names given in Column I with the IUPAC names given in Column II.
| Column l (Common names) |
Column ll (IUPAC names) |
| A. Cinnamaldehyde | 1. Pentanal |
| B. Acetophenone | 2. Prop-2-enal |
| C. Valeraldehyde | 3. 1-Phenylethanone |
| D. Acrolein | 4. 3-Phenylprop-2-en-al |
Codes:
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 1 | 2 |
Match the acids given in Column I with their correct IUPAC names given in Column II and mark the appropriate option:
| Column l (Acids) |
Column ll (IUPAC names) |
| A. Phthalic acid | 1. Hexane-1,6-dioic acid |
| B. Glutaric acid | 2. Benzene-1,2-dicarboxylic acid |
| C. Succinic acid | 3. Pentane-1,5-dioic acid |
| D. Adipic acid | 4. Butane-1,4-dioic acid |
Codes:
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 2 | 1 |
The reaction that does not give benzoic acid as the major product is:
| 1. |  \(\xrightarrow{K_2Cr_2O_7}\) \(\xrightarrow{K_2Cr_2O_7}\) |
2. |  \(\xrightarrow[(ii)H_3O^+]{(i)NaOCl}\) \(\xrightarrow[(ii)H_3O^+]{(i)NaOCl}\) |
| 3. |  \(\xrightarrow{PCC}\) \(\xrightarrow{PCC}\) |
4. |  \(\xrightarrow{KMnO_4/H^+}\) \(\xrightarrow{KMnO_4/H^+}\) |




