Select Chapter Topics:
Which of the following compounds is most prone to oxidation?
1.
\(\mathrm{CH}_3-\mathrm{CHOH}-\mathrm{CH}_3\)2.

3.
\(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3\)4.
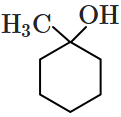
\(\mathrm{CH}_3-\mathrm{CHOH}-\mathrm{CH}_3\)

\(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3\)

Subtopic: Alcohols: Preparation & Properties |
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The reagent used in the conversion of the following reaction is:
| 1. | \(\mathrm{NaBH}_4 \) | 2. | \(\mathrm{LiAlH}_4 \) |
| 3. | \(PCC \) | 4. | \(\mathrm{KMnO}_4\) |
Subtopic: Alcohols: Preparation & Properties |
81%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The product (A) in the below mentioned reaction is:


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Alcohols: Preparation & Properties |
64%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
Links
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The structure of A in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Alcohols: Preparation & Properties |
85%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The reducing agent among the following is -
1. CrO3/H+
2. KMnO4
3. LiAlH4
4. O3
Subtopic: Alcohols: Preparation & Properties |
89%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Optically active 2-octanol rapidly loses its optical activity when exposed to :
| 1. | Dilute acid | 2. | Dilute base |
| 3. | Light | 4. | Humidity |
Subtopic: Alcohols: Preparation & Properties | Mechanism of Dehydration, Methanol & Ethanol |
58%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Product (A) in the reaction below is:


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Phenols: Preparation & Properties |
72%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
Links
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
An ester cannot be prepared by -
1. RCOOH + R'OH + OH-
2. RCOCl+R'OH +Pyridine
3. RCOOH+ R’OH +H+
4. (RCO)2O + R'OH + Pyridine
Subtopic: Alcohols: Preparation & Properties |
50%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Widespread deaths due to liquor poisoning occur due to the presence of-
| 1. | Lead compounds in liquor | 2. | Methyl alcohol in liquor |
| 3. | Ethyl alcohol in liquor | 4. | Carbonic acid in liquor |
Subtopic: Mechanism of Dehydration, Methanol & Ethanol |
78%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital













