Compounds I and II can be distinguished by using reagent:
(I) 4–Amino–2–methylbut–3–en–2–ol
(II) 4–Amino–2,2–dimethylbut–3–yn–1–ol
1. NaNO2/HCl
2. Br2/H2O
3. HCl/ZnCl2 (anhydrous)
4. Cu2Cl2 + NH4OH
(I) 4–Amino–2–methylbut–3–en–2–ol
(II) 4–Amino–2,2–dimethylbut–3–yn–1–ol
What is the correct order of dehydration rate for the compounds (i), (ii), and (iii) when treated with concentrated \(\text{H}_2\text{SO}_4\)?
| (i) |  |
| (ii) |  |
| (iii) |  |
1. (i) > (iii) > (ii)
2. (i) > (ii) > (iii)
3. (ii) > (i) > (iii)
4. (ii) > (iii) > (i)
Ether cannot be produced as a major product by the reaction of:
| 1. | CH3CH2Cl + Ag2O(dry) → |
| 2. | (CH3)3CCl + CH3CH2O-Na+ → |
| 3. |  |
| 4. |  |
The labeled -O18 will be:
1. H2O
2. Methyl benzoate
3. Both 1 and 2
4. Benzoic acid
Product (A) in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following compounds is most prone to oxidation?
| 1. | \(\mathrm{CH}_3-\mathrm{CHOH}-\mathrm{CH}_3\) |
2. |  |
| 3. | \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3\) |
4. | 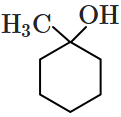 |
| 1. | \(\mathrm{NaBH}_4 \) | 2. | \(\mathrm{LiAlH}_4 \) |
| 3. | \(PCC \) | 4. | \(\mathrm{KMnO}_4\) |

| 1. |  |
2. |  |
| 3. |  |
4. |  |
The structure of A in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |















