Select Chapter Topics:
In the given reaction, product A is:
\(\text{1-Methylcyclohexanol}~ \mathrm{\xrightarrow[]{acid \ catalysed \ dehydration}} ~\text A\)
1. 2-Methylcyclohexene
2. 1-Methylcyclohexanol
3. 1-Methylcyclohexane
4. 1-Methylcyclohexene
\(\text{1-Methylcyclohexanol}~ \mathrm{\xrightarrow[]{acid \ catalysed \ dehydration}} ~\text A\)
1. 2-Methylcyclohexene
2. 1-Methylcyclohexanol
3. 1-Methylcyclohexane
4. 1-Methylcyclohexene
Subtopic: Mechanism of Dehydration, Methanol & Ethanol |
76%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Given below are two statements:
| Assertion (A): | Ortho and para nitrophenols are more acidic than phenol. |
| Reason (R): | Phenoxide ion formed by ortho and para nitrophenols are more stable than pehenoxide ion formed by phenol. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Subtopic: Electrophilic Substitution Reactions, Uses of Phenols |
84%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Consider the two statements given below:
| I. | The given reaction is known as Kolbe’s reaction. |
| II. | The given reaction is known as Reimer - Tiemann reaction. |
1. Both Statement I and Statement II are true.
2. Statement I is true and Statement II is false.
3. Both Statement I and Statement II are false.
4. Statement I is false, Statement II is true.
Subtopic: Phenols: Preparation & Properties |
75%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
In the given reaction, product A and the reaction name are respectively:

| 1. | 2-Ethoxy-3-methylpentane; Williamson synthesis reaction |
| 2. | 1-Ethoxy-3-methylpentane; Williamson synthesis reaction |
| 3. | 2-Ethoxy-3-methylpentane; Nucleophilic addition reaction |
| 4. | 1-Ethoxy-3-methylpentane; Nucleophilic addition reaction |
Subtopic: Ethers: Preparation & Properties, Uses |
80%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene is:
| 1. |  |
| 2. |  |
| 3. | Both 1 and 2 are appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene |
| 4. | None of the above |
Subtopic: Ethers: Preparation & Properties, Uses |
70%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
In the given reaction, products are respectively:
\(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_3+\mathrm{HBr} \rightarrow \mathrm{A}+\mathrm{B}\)
| 1. |  |
| 2. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br} ; \mathrm{CH}_3 \mathrm{OH}\) |
| 3. |  |
| 4. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br} ; \mathrm{CH}_3 \mathrm{Br}\) |
Subtopic: Ethers: Preparation & Properties, Uses |
80%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Match the items in Column I with the items in Column II.
1. a-ii; b-iii; c-i
2. a-ii; b-ii; c-i
3. a-iii; b-i; c-i
4. a-iii; b-ii; c-ii
| Column I | Column II |
a.  |
i. Tertiary alcohol |
| b. H2C=CH−CH2OH | ii. Primary alcohol |
| c.
CH3−CH2−CH2−OH |
iii. Secondary alcohol |
2. a-ii; b-ii; c-i
3. a-iii; b-i; c-i
4. a-iii; b-ii; c-ii
Subtopic: Alcohols: Preparation & Properties |
86%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Which option correctly represents product A?


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Mechanism of Dehydration, Methanol & Ethanol |
76%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
In the given reaction, product A is:


| 1. | 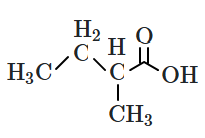 |
2. |  |
| 3. |  |
4. | None of the above. |
Subtopic: Alcohols: Preparation & Properties |
78%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
In the given reaction, product A is:
Butan-1-ol \(\xrightarrow[]{acid \ catalysed \ dehydration}\) A
1. But-2-ene
2. 1-Butoxybutane
3. But-1-ene
4. None of the above
Butan-1-ol \(\xrightarrow[]{acid \ catalysed \ dehydration}\) A
1. But-2-ene
2. 1-Butoxybutane
3. But-1-ene
4. None of the above
Subtopic: Mechanism of Dehydration, Methanol & Ethanol |
61%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital






