When CH3CH2CHCl2 is treated with NaNH2, the product formed is:
1. CH3-CH=CH2
2. CH3-CCH
3. CH3CH2CH
4. CH3CH2C
Chlorobenzene reacts with Mg in dry ether to give a compound (A).
A and ethanol continue to react in order to produce?
| 1. | Phenol | 2. | Benzene |
| 3. | Ethylbenzene | 4. | Phenylether |
R-CH2-CCl2-R R-CC-R. The reagent is
1. Na
2. HCl in H2O
3. KOH in C2H5OH
4. Zn in alcohol
Ethyl chloride is converted into diethyl ether by -
1. Wurtz reaction
2. Grignard reaction
3. Perkin's reaction
4. Williamson's synthesis
The alkyl halide is converted into an alcohol by:
| 1. | Addition | 2. | Substitution |
| 3. | Dehydrohalogenation | 4. | Elimination |
What is produced when Br2 is added to cis-but-2-ene?
1. Racemic mixture of 2,3-Dibromobutane
2. Meso form of 2,3-Dibromobutane
3. Dextro form of 2,3-Dibromobutane
4. Laevo form of 2,3-Dibromobutane
How many unique substitution products can be formed when ethane reacts with bromine under sunlight?
| 1. | 9 | 2. | 6 |
| 3. | 8 | 4. | 5 |
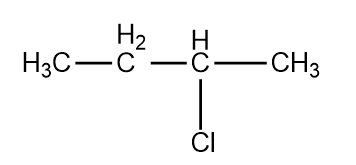 , Cl obtained by chlorination of n-butane, will be
, Cl obtained by chlorination of n-butane, will be
1. meso form
2. racemic mixture
3. d-form
4. l-form
The reactivity order of halides for dehydrohalogenation is
1. R-F>R-Cl>R-Br>R-I
2. R-I>R-Br>R-Cl>R-F
3. R-I>R-Cl>R-Br>R-F
4. R-F>R-I>R-Br>R-Cl
In reactions, the correct order of reactivity for the following compounds : , , and is :
1. >>>
2. >>>
3. >>>
4. >>>






