The relationship between temperature and the variance in reaction rate is:
1.

2.

3.

4.






Half-life is independent of the concentration of a reactant. After 10 minutes, the volume of N2 gas is 10 L and after complete reaction, it is 50 L. Hence, the rate constant is:
1. log 5 min-1
2. log 1.25 min-1
3. log 2 min-1
4. log 4 min-1
Consider the following graph
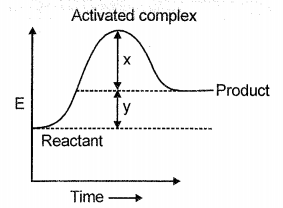
Choose the incorrect statement(s)
1. Reaction is exothermic forward direction i.e., H = -ve
2. Activation energy for forward reaction is x - y
3. Using catalyst energy will decrease H value of the reaction
4. All of these
The half-life for radioactive decay of 14C is 5730 years. A wood sample contains only 80% of the 14C. The age of the wood sample would be-
1. 1898 years
2. 1765 years
3. 1931 years
4. 1860 years
Sucrose hydrolysis in an acidic solution into glucose and fructose follows the first-order rate law with a half-life of 3.33 h at 25°C. After 9 h, the fraction of sucrose remaining is f. The value of \(log(\frac{1}{f})\) is A×10-2 . The value of A is:
(Rounded off to the nearest integer) [Assume : ln 10 = 2.303, ln 2 = 0.693]
1. 78
2. 81
3. 85
4. 75
Consider the reaction, 2A + B → Products.
When concentration of B alone was doubled, the half-life did not change. When the concentration of A alone was doubled, the rate increased by two times. The unit of rate constant for this reaction is:
1. L mol–1 s–1
2. no unit
3. mol L–1s–1
4. s–1
1. 1.61
2. 1.41
3. 1.81
4. 1.21
1. 2.67 × 10−19
2. 3.57 × 10−18
3. 4.67 × 10−17
4. 1.47 × 10−19
Reason(R): By using a suitable catalyst, we can increase the rate of reaction.
1. Both A and R are correct and R is the correct explanation of A.
2. Both A and R are correct but R is not the correct explanation of A.
3. A is true and R is false
4. A is false and R is true
| Assertion(A): | For exothermic reaction equilibrium constant decreases with an increase in temperature. |
| Reason(R): | For a reaction, the rate constant decreases with decrease in temperature. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True and (R) is False |
| 4. | (A) is False and (R) is True |






