is more stable than because :
| 1. | NO2 shows +I effect |
| 2. | NO2 shows -I effect |
| 3. | NO2 decreases the positive charge on the compound |
| 4. | Ethyl group increases positive charge on the compound |
The effect that makes 2,3–dimethyl-2-butene more stable than 2-butene is-
1. Resonance
2. Hyperconjugation
3. Steric effect
4. Inductive effect
The most stable carbocation among the following is-
1. \(({CH_3})_3C\overset{+}{C}H_2\)
2. \(({CH_3})_3\overset{+}{C}\)
3. \({CH_3}CH_2\overset{+}{C}H_2\)
4. \(({CH_3})\overset{+}{C}HCH_2CH_3\)
Which carboxylate ion among the following is the most stable?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Compare the stability of the two resonating structures given below and mark the correct option:
1. (I) is more stable than (II)
2. (II) is more stable than (I)
3. (I) and (II) both have the same stability
4. None of the above
The carbocation among the following that doesn't get stabilized by resonance is :
| 1. | 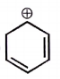 |
2. | 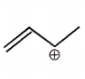 |
| 3. | 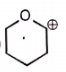 |
4. | 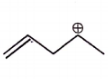 |
The total number of resonating structures (excluded the given structure) formed by the given molecule are :
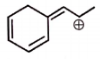
| 1. | 2 | 2. | 3 |
| 3. | 4 | 4. | 5 |
The correct order with respect to –I effect of the substituents is:
(R = alkyl)
1. –NH2 > –OR < –F
2. –NR2 < –OR < –F
3. –NH2 > –OR > –F
4. –NR2 > –OR > –F





