The correct hybridization states of carbon atoms in the following compound are -
| 1. | C1= sp , C2= sp3 , C3= sp2 | 2. | C1= sp2 , C2= sp3 , C 3 = sp3 |
| 3. | C1= sp2 , C2= sp2 , C 3 = sp | 4. | C1= sp3 , C2= sp3 , C 3 = sp3 |
The number of σ and π bonds in the molecule are -
| 1. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 2. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
| 3. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 4. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
The number of primary carbon atoms in the following compound are:

| 1. | 6 | 2. | 2 |
| 3. | 4 | 4. | 3 |
The \(C - H\) bond distance is longer in -
| 1. | \(C_2H_2\) | 2. | \(C_2H_4\) |
| 3. | \(C_2H_6\) | 4. | \(C_2H_2Br_2\) |

| 1. | 4 | 2. | 8 |
| 3. | 12 | 4. | 16 |
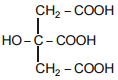
The IUPAC name of the above mentioned compound is -
1. Citric acid
2. 3-Hydroxy pentane-1,5-dioic acid
3. 2-Hydroxypropane-1,2,3-tricarboxylic acid
4. 2-Carboxy-2-hydroxy propane-1,3-dicarboxylic acid
1. 2–Bromo-3–methylbutanoic acid
2. 2-Methyl-3-bromobutanoic acid
3. 3-Bromo-2-methylbutanoic acid
4. 3-Bromo-2,3-dimethylpropanoic acid.
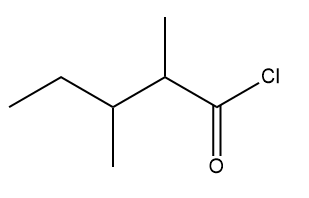
The IUPAC name of the above mentioned compound is -
1. 3, 4-Dimethylpentanoyl chloride
2. 1-Chloro-1-oxo-2,3-dimethylpentane
3. 2-Ethyl-3-methylbutanoylchloride
4. 2, 3-Dimethylpentanoyl chloride
The correct IUPAC name, among the following, is:
| 1. | Prop-3-yn-1-ol | 2. | But-4-ol-4-yne |
| 3. | But-3-ol-2-yne | 4. | But-3-yn-1-ol |

The IUPAC name of the above-mentioned compound is:
1. Pent-1-en-3-yne
2. Pent-1-ene-4-yne
3. Pent-4-yn-1-ene
4. Pent-1-en-4-yne







