Arrange H, CH₃, C₂H₅, and C₃H₇ in order of increasing positive inductive effect:
1. H < CH3 < C2H5 < C3H7
2. H> CH3 <C2H5 > C3H7
3. H < C2H5 < CH3 <C3H7
4. None of the above
The effect that can explain the given order of acidity of the carboxylic acids is-
Cl3CCOOH > Cl2CHCOOH > ClCH2COOH
1. +I effect
2. -I effect
3. +E effect
4. -E effect
is more stable than because :
| 1. | NO2 shows +I effect |
| 2. | NO2 shows -I effect |
| 3. | NO2 decreases the positive charge on the compound |
| 4. | Ethyl group increases positive charge on the compound |
The effect that makes 2,3–dimethyl-2-butene more stable than 2-butene is-
1. Resonance
2. Hyperconjugation
3. Steric effect
4. Inductive effect
Among the following groups maximum –I effect is exerted by:
\(1 . - C_{6} H_{5}\)
\(2 .\) \(- \left(OCH\right)_{3}\)
\(3 .\) \(- Cl\)
\(4 .\) \(-NO_2\)
The most stable carbocation among the following is-
1. \(({CH_3})_3C\overset{+}{C}H_2\)
2. \(({CH_3})_3\overset{+}{C}\)
3. \({CH_3}CH_2\overset{+}{C}H_2\)
4. \(({CH_3})\overset{+}{C}HCH_2CH_3\)
The most stable carbocation among the following is-
| 1. | \(({CH_3})_3C\overset{+}{C}HCH_3\) | 2. | \({CH_3}CH_2\overset{+}{C}HCH_2CH_3\) |
| 3. | \(({CH_3})_2\overset{+}{C}CH_2CH_2CH_3\) | 4. | \({CH_3}CH_2\overset{+}{C}H_2\) |
Which carboxylate ion among the following is the most stable?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Compare the stability of the two resonating structures given below and mark the correct option:
1. (I) is more stable than (II)
2. (II) is more stable than (I)
3. (I) and (II) both have the same stability
4. None of the above
The carbocation among the following that doesn't get stabilized by resonance is :
| 1. | 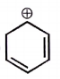 |
2. | 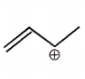 |
| 3. | 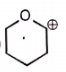 |
4. | 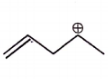 |







