For the following equilibrium, Kc= 6.3 × 1014 at 1000 K
The value of Kc for the reverse reaction is:
The value of Kc for the reverse reaction is:
\(\mathrm{K}_{\mathrm{c}}=\frac{\left[\mathrm{NH}_3\right]^4\left[\mathrm{O}_2\right]^5}{[\mathrm{NO}]^4\left[\mathrm{H}_2 \mathrm{O}]^6\right.}\)
The balanced chemical equation corresponding to the above-mentioned expression is:
| 1. | \(4 \mathrm{NO}_{(\mathrm{g})}+6 \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})} \rightleftharpoons 4 \mathrm{NH}_{3(\mathrm{g})}+5 \mathrm{O}_{2(\mathrm{g})} \) |
| 2. | \(4 \mathrm{NH}_3(\mathrm{g})+5 \mathrm{O}_{2(\mathrm{g})} \rightleftharpoons 4 \mathrm{NO}_{(\mathrm{g})}+6 \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})}\) |
| 3. | \(\ 2 \mathrm{NO}_{(\mathrm{g})}+3 \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})} \rightleftharpoons 4 \mathrm{NH}_{3(\mathrm{g})}+3 \mathrm{O}_{2(\mathrm{g})}\) |
| 4. | \(\ \mathrm{NH}_{3(\mathrm{g})}+3 \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})} \rightleftharpoons 2 \mathrm{NO}_{(\mathrm{g})}+3 \mathrm{O}_{2(\mathrm{g})}\) |
One mole of H2O and
one mole of CO are taken in a 10 L vessel and heated to
725 K. At equilibrium, 40% of water(by mass) reacts with CO according to the equation,
H2O (g) + CO (g) H2 (g) + CO2 (g)
The equilibrium constant for the above-mentioned reaction would be:
| 1. | 0.66 | 2. | 0.35 |
| 3. | 0.44 | 4. | 0.82 |
The equilibrium pressure of C2H6 when it is placed in a flask at 4.0 atm pressure at 899 K would be:
C2H6 (g) C2H4 (g) + H2 (g)
( Kp = 0.04 atm at 899 K)
| 1. | 4.12 atm | 2. | 3.62 atm |
| 3. | 1.54 atm | 4. | 2.16 atm |
A sample of pure PCl5 was introduced into an evacuated vessel at 473 K.
After equilibrium was attained, a concentration of PCl5
was found to be 0.5 × 10–1 mol L–1. If the value of
Kc is 8.3 × 10–3 mol L–1, the concentrations of
PCl3 and Cl2 at equilibrium would be:
PCl5 (g) PCl3 (g) + Cl2(g)
| 1. | |
| 2. | |
| 3. | |
| 4. |
Match the following equilibria with the corresponding condition.
| A. LiquidVapour | 1. Saturated solution |
| B. SolidLiquid | 2. Boiling point |
| C. SolidVapour | 3. Sublimation point |
| D. Solute (s)Solute (solution) | 4. Melting point |
| 5. Unsaturated solution |
Codes
| A | B | C | D | |
| 1. | 2 | 4 | 3 | 1 |
| 2. | 1 | 2 | 3 | 5 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 3 | 2 |
Some reactions are written below in Column I and their equilibrium constants
in terms of Kc are written in Column II.
Match the following reactions with the corresponding equilibrium constant.
| Column I (Reaction) | Column II (Equilibrium constant) |
| A. | 1. |
| B. | 2. |
| C. | 3. |
| 4. |
Codes
| A | B | C | |
| 1. | 4 | 3 | 2 |
| 2. | 1 | 2 | 3 |
| 3. | 1 | 4 | 3 |
| 4. | 4 | 1 | 3 |
Match the standard free energy of the reaction with the corresponding equilibrium constant.
| A. | 1. K>1 |
| B. | 2. K=1 |
| C. | 3. K=0 |
| 4. K<1 |
Codes
| A | B | C | |
| 1. | 4 | 1 | 2 |
| 2. | 1 | 2 | 3 |
| 3. | 2 | 4 | 3 |
| 4. | 4 | 1 | 3 |
Match the following species with the corresponding conjugate acid.
| Species | Conjugate acid |
| A. | 1. |
| B. | 2. |
| C. | 3. |
| D. | 4. |
| 5. |
Codes
| A | B | C | D | |
| 1. | 2 | 5 | 1 | 5 |
| 2. | 2 | 4 | 3 | 5 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 3 | 2 |
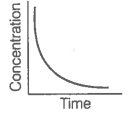
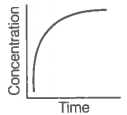
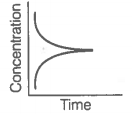
|
A |
B |
|
A. |
i. Variation in product concentration with time. |
|
B. |
ii. Reaction at equilibrium |
|
C. |
iii. Variation in reactant concentration with time. |
Match the graphical variations with their descriptions given above and identify the correct codes below:
| A | B | C | |
| 1. | i | iii | ii |
| 2. | i | ii | iii |
| 3. | iii | ii | i |
| 4. | iii | i | ii |