The molecules having dipole moment are ________
a. 2,2-Dimethylpropane
b. trans-Pent-2-ene
c. cis-Hex-3-ene
d. 2,2,3,3 - Tetramethylbutane
Choose the correct option
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Which of the following is/are aromatic structure(s)?
| a. |  |
b. |  |
| c. |  |
d. |  |
| 1. | a and b | 2. | b and c |
| 3. | c and d | 4. | a and c |
Correct statement among the following is:
| a. | CH3-O-CH2⊕ is more stable than CH3-CH2⊕ |
| b. | (CH3)2CH⊕ is less stable than CH3-CH2-CH2⊕ |
| c. | CH2=CH-CH2⊕ is more stable than CH3-CH2-CH2⊕ |
| d. | CH2 = CH⊕ is more stable than CH3-CH2⊕ |
Choose the correct option
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
What is the impact of the nitro substituent on the reactivity and orientation of electrophilic substitution in nitrobenzene?
| (a) | Deactivates the ring by inductive effect. |
| (b) | Activates the ring by inductive effect. |
| (c) | Decreases the charge density at the ortho and para positions of the ring relative to the meta position by resonance. |
| (d) | Increases the charge density at the meta position relative to the ortho and the para positions of the ring by resonance. |
Choose the correct option:
1. a and b
2. b and c
3. c and d
4. a and c
For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring:
| A | Deactivates the ring by inductive effect |
| B | Deactivates the ring by resonance |
| C | Increases the charge density at the ortho and the para positions relative to the meta position by resonance. |
| D | Directs the incoming electrophile to the meta position by increasing the charge density relative to the ortho and the para positions. |
Choose the correct option:
| 1. | A and B | 2. | B and C |
| 3. | C and D | 4. | A and C |
Which are the IUPAC names of the following compounds?
a. 5-(2',2'-Dimethylpropyl)decane
b. 4-Butyl-2,2-dimethylnonane
c. 2,2-Dimethyl -4- pentyloctane
d. 5-Neopentyldecane
Choose the correct option
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, d) |
Which are the correct IUPAC names of the following compound?

a. 5-Butyl-4-isoporpyldecane
b. 5-Ethyl-4-propyldecane
c. 5-sec-Butyl -4- iso-propyldecane
d. 4-(1-Methylethyl) - 5 - (1-methylpropyl)decane
Choose the correct option
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, c) |
Which of the following alkenes undergo ozonolysis to produce a mixture of ketones only?
| a. |  |
b. | 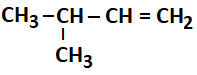 |
| c. |  |
d. |  |
Choose the correct option:
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, c) |
Some oxidation reactions of methane are given below. Which of them is/are controlled oxidation reactions?
| a. | CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) |
| b. | CH4(g) + O2(g) → C(s) + 2H2O(l) |
| c. | CH4(g) + O2(g) \(\xrightarrow[]{Mo_{2}O_{3}}\) HCHO + H2O |
| d. | 2CH4(g) + O2(g) \(\xrightarrow[]{Cu/523 \ K/ 100 \ atm}\)2CH3OH |
Choose the correct option
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Given below are two statements:
| Assertion (A): | Among isomeric pentanes, 2,2-dimethylpropane has the highest boiling point. |
| Reason (R): | Branching does not affect the boiling point. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | Both (A) and (R) are False. |
| 4. | (A) is True but (R) is False. |







