Which pair of ions from the following list do not constitute an iso-electronic pair?
1. Mn2+, Fe3+
2. Fe2+, Mn2+
3. O2-, F-
4. Na+, Mg2+
1. Mn2+, Fe3+
The following graph captures potential energy on the y-axis for hydrogen gas formation as a function of the internuclear distance on the x-axis:
The bond energy of H2 can be represented by-
| 1. | (c – a) | 2. | (b – a) |
| 3. | (c-a)/2 | 4. | (b-a)/2 |
The correct Lewis structure of acetic acid is:
| 1. |  |
2. |  |
| 3. |  |
4. | None of the above. |
The significance of the octet rule is:
1. To determine the stability of atoms.
2. To find the hybridization of elements.
3. To calculate the number of lone pairs.
4. None of the above.
The lone pairs of electrons can be defined as -
1. Electron pairs that participate in bonding.
2. Electron pairs that do not participate in bonding.
3. Electron pairs that are present in inner most shell.
4.Electron pairs that are present in valence shell of ions.
CH4 does not exhibit square planar geometry because:
| 1. | For square planar geometry, 5 bonds are required. |
| 2. | Carbon does not have d-orbitals to undergo dsp2 hybridization. |
| 3. | Due to steric hindrance CH4 does not exhibit square planar geometry. |
| 4. | Carbon does not have d-orbitals to undergo d2sp3 hybridization. |
The application of dipole moment is/are :
| 1. | It is used to differentiate polar and non-polar bonds. |
| 2. | It is helpful in calculating the percentage ionic character of a molecule. |
| 3. | Both '1' and '2' |
| 4. | None of the above. |
can be represented by structures 1 and 2 shown below.
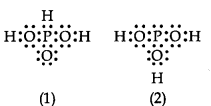
These two structures cannot be taken as the canonical forms of the resonance hybrid, because :
1. The positions of the atoms have changed.
2. The positions of the atoms are constant.
3. H3PO3 does not show resonance.
4. Two hydrogen atoms are missing.
The bond length can be defined as -
| 1. | The equilibrium distance between the nuclei of two bonded atoms in a molecule. |
| 2. | The farthest distance between the nuclei of two bonded atoms in a molecule. |
| 3. | The shortest distance between the nuclei of two bonded atoms in a molecule. |
| 4. | None of the above. |
The favourable factors for the formation of an ionic bond would be:
| 1. | Low ionization enthalpy, High negative electron gain enthalpy, High lattice energy. |
| 2. | High ionization enthalpy, High electron gain enthalpy, High lattice energy. |
| 3. | Low ionization enthalpy, Low electron gain enthalpy, Low lattice energy. |
| 4. | High ionization enthalpy, Low electron gain enthalpy, High lattice energy. |



