Which of the following diagrams (figure) depicts ideal gas behaviour?
1. (a), (c)
2. (a), (d)
3. (c), (d)
4. (a), (b)
Boyle's law is applicable for an:
| 1. | adiabatic process |
| 2. | isothermal process |
| 3. | isobaric process |
| 4. | isochoric process |
A cylinder containing an ideal gas is in a vertical position and has a piston of mass M that is able to move up or down without friction (figure). If the temperature is increased,
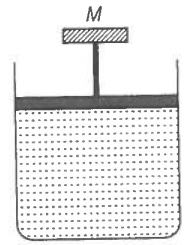
1. both P and V of the gas will change.
2. only P will increase according to Charles' law.
3. V will change but not P.
4. P will change but not V.
The volume versus temperature graphs for a given mass of an ideal gas are shown in the figure at two different values of constant pressure. What can be inferred about relation between \(\mathrm{P_1}\) and \(\mathrm{P_2}\)?
1. \(\mathrm{P_1}>\mathrm{P_2} \)
2. \(\mathrm{P_1}=\mathrm{P_2} \)
3. \(\mathrm{P_1}<\mathrm{P_2} \)
4. data is insufficient
1 mole of gas is contained in a box of volume V = 1.00 at T = 300 K. The gas is heated to a temperature of T = 3000 K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be: (considering all gases to be ideal)
1. same as the pressure initially
2. 2 times the pressure initially
3. 10 times the pressure initially
4. 20 times the pressure initially
An inflated rubber balloon contains one mole of an ideal gas, has a pressure \(P,\) volume \(V\) and temperature \(T.\) If the temperature rises to \(1.1T,\) and the volume is increased to \(1.05V,\) the final pressure will be:
| 1. | \(1.1P\) |
| 2. | \(P\) |
| 3. | less than \(P\) |
| 4. | between \(P\) and \(1.1P\) |




