A monoatomic ideal gas, initially at temperature \(T_1\), is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature \(T_2\) by releasing the piston suddenly. If \(L_1\) and \(L_2\) are the lengths of the gas column before and after expansion, respectively, then \(\frac{T_1}{T_2}\) is given by:
1. \(\left(\frac{L_1}{L_2}\right)^{\frac{2}{3}}\)
2. \(\frac{L_1}{L_2}\)
3. \(\frac{L_2}{L_1}\)
4. \(\left(\frac{L_2}{L_1}\right)^{\frac{2}{3}}\)
1. \(\left(\frac{L_1}{L_2}\right)^{\frac{2}{3}}\)
2. \(\frac{L_1}{L_2}\)
3. \(\frac{L_2}{L_1}\)
4. \(\left(\frac{L_2}{L_1}\right)^{\frac{2}{3}}\)
The initial pressure and volume of a gas are \(P\) and \(V\), respectively. First, it is expanded isothermally to volume \(4V\) and then compressed adiabatically to volume \(V\). The final pressure of the gas will be: [Given: \(\gamma = 1.5\)]
| 1. | \(P\) | 2. | \(2P\) |
| 3. | \(4P\) | 4. | \(8P\) |
If \(n\) moles of an ideal gas is heated at a constant pressure from \(50^\circ\text C\) to \(100^\circ\text C,\) the increase in the internal energy of the gas will be:
\(\left(\frac{C_{p}}{C_{v}} = \gamma\ ~\text{and}~\ R = \text{gas constant}\right)\)
| 1. | \(\dfrac{50nR}{\gamma - 1}\) | 2. | \(\dfrac{100nR}{\gamma - 1}\) |
| 3. | \(\dfrac{50n\gamma R}{\gamma - 1}\) | 4. | \(\dfrac{25n\gamma R}{\gamma - 1}\) |
1. \(\frac{\eta}{2}\)
2. \(\eta\)
3. \(2\eta\)
4. \(3\eta\)
The latent heat of vaporisation of water is \(2240~\text{J/gm}\). If the work done in the process of expansion of \(1~\text{g}\) is \(168~\text{J}\),
then the increase in internal energy is:
1. \(2408~\text{J}\)
2. \(2240~\text{J}\)
3. \(2072~\text{J}\)
4. \(1904~\text{J}\)
The figure below shows two paths that may be taken by a gas to go from state A to state C. In process AB, \(400~\text{J}\) of heat is added to the system and in process BC, \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process AC will be:
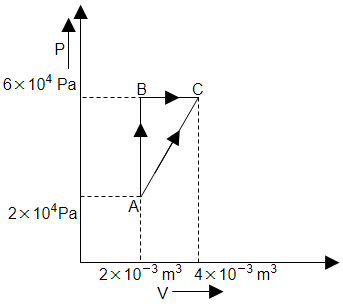
| 1. | \(380~\text{J}\) | 2. | \(500~\text{J}\) |
| 3. | \(460~\text{J}\) | 4. | \(300~\text{J}\) |
The first law of thermodynamics is based on:
| 1. | the concept of temperature. |
| 2. | the concept of conservation of energy. |
| 3. | the concept of working of heat engine. |
| 4. | the concept of entropy. |
The efficiency of an ideal heat engine is less than \(100\%\) because of:
| 1. | the presence of friction. |
| 2. | the leakage of heat energy. |
| 3. | unavailability of the sink at zero kelvin. |
| 4. | all of these. |
Work done during the given cycle is:
1. 4
2. 2
3.
4.
An ideal gas goes from \(A\) to \(B\) via two processes, \(\mathrm{I}\) and \(\mathrm{II},\) as shown. If \(\Delta U_1\) and \(\Delta U_2\) are the changes in internal energies in processes \(\mathrm{I}\) and \(\mathrm{II},\) respectively, (\(P:\) pressure, \(V:\) volume) then:

| 1. | \(∆U_1 > ∆U_2\) | 2. | \(∆U_1 < ∆U_2\) |
| 3. | \(∆U_1 = ∆U_2\) | 4. | \(∆U_1 \leq ∆U_2\) |





