The correct statement based on the graph below is:
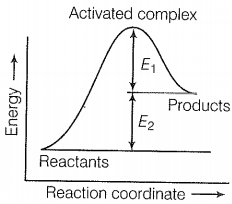
1.
The activation energy of the forward reaction is E1 + E2 and the product is less stable than reactant.
2.
The activation energy of the forward reaction is E1 + E2 and the product is more stable than the reactant.
3.
The activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than the product.
4.
The activation energy of the backward reaction is E1 and the product is more stable than reactant.

The correct graphical representation of relation between ln k and 1/T is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in the graph below.
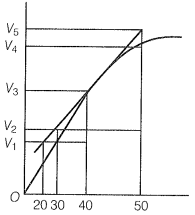
The correct statement among the following based on the graph given above is:
The correct representation of an exothermic reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. | Both 1 and 2 |
Consider the reaction AB. The concentration of both the reactant and the product varies exponentially with time.
The graph that accurately depicts how reactant and product concentrations change with time is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The correct statements among the following regarding a balanced chemical equation of an elementary reaction are-
| a. | Order is the same as molecularity. |
| b. | Order is less than the molecularity. |
| c. | Order is greater than molecularity. |
| d. | Molecularity can never be zero. |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
The correct statements among the following regarding any unimolecular reaction are-
| a. | Only one reacting species is involved in the rate-determining step |
| b. | The order and the molecularity of the slowest step are equal to one |
| c. | The molecularity of the reaction is one and the order is zero |
| d. | Both molecularity and order of the reaction are one |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
The correct statements among the following regarding a complex reaction are-
| a. | Order of the overall reaction is the same as the molecularity of the slowest step. |
| b. | Order of the overall reaction is less than the molecularity of the slowest step. |
| c. | Order of the overall reaction is greater than the molecularity of the slowest step. |
| d. | The molecularity of the slowest step is never zero or non-integer. |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
At high pressure the following reaction is zero order-
The correct statements among the following is:
| a. | Rate of reaction = Rate constant |
| b. | Rate of the reaction depends on the concentration of ammonia |
| c. | Rate of decomposition of ammonia will remain constant until ammonia disappears completely |
| d. | Further increase in pressure will change the rate of reaction |
| 1. | (a, b, c) | 2. | (b, c, d) |
| 3. | (a, c, d) | 4. | (a, b, d) |
The correct statements among following about Maxwell, Boltzmann distribution of energy is -
| a. | The fraction of molecules with the most probable kinetic energy decreases at higher temperatures |
| b. | The fraction of molecules with the most probable kinetic energy increases at higher temperatures |
| c. | Most probable kinetic energy increases at higher temperatures |
| d. | Most probable kinetic energy decreases at higher temperatures |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)



