Select the correct option based on statements below:
Assertion (A):
For elementary reactions, the law of mass action and the rate of law expression are generally the same.
Reason (R):
The molecularity of an elementary reaction is always one.
1.
Both (A) and (R) are true and (R) is the correct explanation of (A).
2.
Both (A) and (R) are true but (R) is not the correct explanation of (A).
3.
(A) is true but (R) is false.
4.
Both (A) and (R) are false.
Select the correct option based on statements below:
| Assertion (A): | At very high temperatures (approaches to infinity), the rate constant becomes equal to the collision frequency. |
| Reason (R): | The collision in which molecules collide with proper orientation is called an ineffective collision. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Select the correct option based on statements below:
| Assertion (A): | Base catalyzed hydrolysis of ethyl acetate is a first-order reversible reaction. |
| Reason (R): | The order of reaction always depends on the stoichiometry of the reaction. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Select the correct option based on statements below:
| Assertion (A): | The overall order of reaction is the sum of the power of all the reactants in the rate expression. |
| Reason (R): | There are many higher-order reactions. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
|
A |
B |
|
A. |
i. Variation in product concentration with time. |
|
B. |
ii. Reaction at equilibrium |
|
C. |
iii. Variation in reactant concentration with time. |
Match the graphical variations with their descriptions given above and identify the correct codes below:
| A | B | C | |
| 1. | i | iii | ii |
| 2. | i | ii | iii |
| 3. | iii | ii | i |
| 4. | iii | i | ii |
The correct statement based on the graph below is:

| 1. | The activation energy of the forward reaction is E1 + E2 and the product is less stable than reactant. |
| 2. | The activation energy of the forward reaction is E1 + E2 and the product is more stable than the reactant. |
| 3. | The activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than the product. |
| 4. | The activation energy of the backward reaction is E1 and the product is more stable than reactant. |
The correct graphical representation of relation between ln k and 1/T is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in the graph below.

The correct statement among the following based on the graph given above is:
The correct representation of an exothermic reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. | Both 1 and 2 |
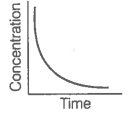
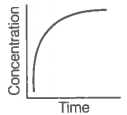
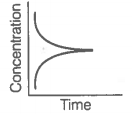
Consider the reaction AB. The concentration of both the reactant and the product varies exponentially with time.
The graph that accurately depicts how reactant and product concentrations change with time is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |