A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in the graph below.
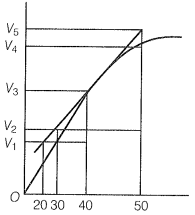
The correct statement among the following based on the graph given above is:

Which of the following statements is not correct about the order of a reaction?
| 1. | The order of a reaction can be a fractional number |
| 2. | Order of a reaction is experimentally determined quantity |
| 3. | The order of a reaction is always equal to the sum of the stoichiometric coefficients of reactants in the balanced chemical equation for a reaction |
| 4. | The order of a reaction is the sum of the powers of the molar concentration of the reactants in the rate law expression |
Consider the graph given in the figure. Which of the following options does not show an instantaneous rate of reaction in the 40s?
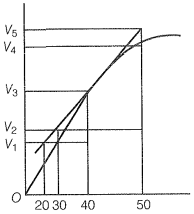
True statement among the following is:
| 1. | The rate of a reaction decreases with the passage of time as the concentration of reactants decreases. |
| 2. | The rate of a reaction is the same at any time during the reaction. |
| 3. | The rate of a reaction is independent of temperature change. |
| 4. | The rate of a reaction decreases with an increase in the concentration of the reactants. |
The correct expression for the rate of reaction given below is:
\(5 \mathrm{Br}^{-}(\mathrm{aq})+\mathrm{BrO}_3^{-}(\mathrm{aq})+6 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 3 \mathrm{Br}_2(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
| 1. | \(\frac{\Delta\left[B r^{-}\right]}{\Delta t}=5 \frac{\Delta\left[H^{+}\right]}{\Delta t} \) | 2. | \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{6}{5} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t} \) |
| 3. | \(\frac{\Delta[\mathrm{Br^-}]}{\Delta t}=\frac{5}{6} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t} \) | 4. | \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=6 \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\) |
The correct representation of an exothermic reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. | Both 1 and 2 |
Rate law for the reaction \(A+2 B \rightarrow C\) is found to be
Rate = k[A][B]
If the concentration of reactant 'B' is doubled, keeping the concentration of A constant, then the value of the rate of the reaction will be:
| 1. | The same. | 2. | Doubled. |
| 3. | Quadrupled. | 4. | Halved. |
An incorrect statement about the collision theory of chemical reaction is:
| 1. | It considers reacting molecules or atoms to be hard spheres and ignores their structural features. |
| 2. | The number of effective collisions determines the rate of reaction. |
| 3. | The collision of atoms or molecules possessing sufficient threshold energy results in product formation. |
| 4. | Molecules should collide in the proper orientation for the collision to be effective with sufficient threshold energy and proper orientation. |
A first-order reaction is 50 % completed in 1.26 x 1014 s.
The time required for 100 % completion
of the reaction will be:
1. 1.26 × 1015 s
2. 2.52 × 1014 s
3. 2.52 × 1028 s
4. Infinite
Compounds A and B react according to the following chemical equation.
A(g) + 2B(g) 2C(g)
The concentration of either A or B was changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. The following results were obtained. Choose the correct option for the rate equations for this reaction.
| Experiment | Initial concentration of [A]/mol L-1 |
Initial concentration of [B]/moI L-1 |
Initial rate (mol L-1 s-1) |
| 1. 2. 3. |
0.30 0.30 0.60 |
0.30 0.60 0.30 |
0.10 0.40 0.20 |


