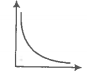
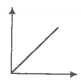
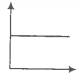
The SI unit of viscosity coefficient is-
1. Pascal
2. Nsm-2
3. km-2s
4. Nm-2
Atmospheric pressure recorded in different cities are as follows
| Cities | p in N/m2 |
| Shimla | |
| Bangalore | |
| Delhi | |
| Mumbai |
The liquid will boil first at -
1. Shimla
2. Bangalore
3. Delhi
4. Mumbai
In the given figure, the curve representing an ideal gas is-
1. B only
2. C and D only
3. E and F only
4. A and B only
Increase in kinetic energy can overcome intermolecular forces of attraction. How will increase in temperature effect the viscosity of liquid-
1. Increase
2. No effect
3. Decrease
4. No regular pattern will be followed
With increase in temperature the surface tension of a liquid will:
1. Remains same
2. Decreases
3. Increases
4. No regular pattern is followed
Match the graphs between the following variables with their names.
|
Graphs |
Names |
|
A. Pressure vs temperature graph at constant molar volume |
1. Isotherms |
|
B. Pressure vs |
2. Constant temperature Curve |
|
C. Volume vs temperature graph at constant pressure. |
3. Isochores |
|
4. Isobars |
Codes
| Options: | A | B | C |
| 1. | 3 | 1 | 4 |
| 2. | 1 | 2 | 3 |
| 3. | 5 | 4 | 3 |
| 4. | 4 | 5 | 3 |
Match the following gas law with the equation representing them.
|
A. Boyle’s law |
1. at constant T and p |
|
B. Charle’s law |
2. …constant T,V |
|
C. Dalton’s law |
3. constant |
|
D. Avogadro’s law |
4. at constant n and p |
|
5. at constant n and T |
Codes
| Options: | A | B | C | D |
| 1. | 5 | 4 | 2 | 1 |
| 2. | 3 | 4 | 2 | 1 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 3 | 2 |
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
| Options: | A | B | C |
| 1. | 2 | 3 | 1 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 2 | 1 |
| 4. | 1 | 3 | 1 |
Given below are two statements:
| Assertion (A): | Three states of matter are the result of a balance between intermolecular forces and the thermal energy of the molecules. |
| Reason (R): | Intermolecular forces tend to keep the molecules together but the thermal energy of molecules tends to keep them apart. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Given below are two statements:
| Assertion (A): | At constant temperature, pV vs p plot for real gases is not a straight line. |
| Reason (R): | At high pressure, all gases have Z>1 but at an intermediate pressure most gases have Z<1. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |