Select Chapter Topics:
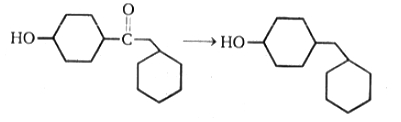
The above reduction can be best carried out by:
1. Clemmensen reduction
2. Wolff-Kishner reduction
3.
4. None of the above

Subtopic: Aldehydes & Ketones: Preparation & Properties |
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
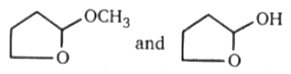
Above compounds can be differentiated by following reagent:
1. 2-4 DNP (Brady reagent)
2. Tollen's reagent
3. Bromine water reagent
4. NaHSO3
Subtopic: Aldehydes & Ketones: Preparation & Properties |
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The following reaction gives
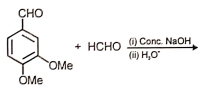
1. 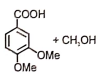 2.
2. 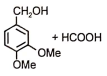
3. 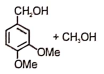 4.
4. 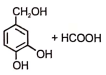
Subtopic: Carboxylic Acids: Preparation & Properties |
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The values of some of the acids are given below:
| Acid | \(\mathrm{pK_a}\) | Acid | \(\mathrm{pK_a}\) |
 |
-0.6 | HI | -10.0 |
 |
4.8 | \(\mathrm{HC} \equiv \mathrm{CH}\) | 25 |
| \(\overset{+}{N}H_4\) | 9.4 | \(H_2S\) | 7.0 |
The correct order of leaving tendency of their conjugate bases is:
| 1. | 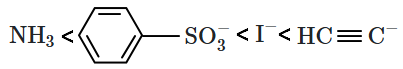 |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Acid Derivatives - Preparation, Properties & Uses |
51%
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Select the correct option based on statements below:
| Assertion (A): | The addition of ammonia derivatives to carbonyl compounds is carried out in a weakly acidic medium. |
| Reason (R): | In a weakly acidic medium, the attacking nucleophile is also protonated. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Subtopic: Name Reaction |
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
[P] on treatment with Br2/FeBr3 in CCl4 produced a single isomer C8H7O2Br while heating [P] with soda lime gave toluene. The compound [P] is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Carboxylic Acids: Preparation & Properties |
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Consider the given statements:
| Statement I: | The conversion of but-2-ene to ethanal can be obtained by reductive ozonolysis. |
| Statement II: | The conversion of allyl alcohol to propenal is a reduction reaction and can be achieved using the PCC reagent. |
| 1. | Statement I is correct; Statement II is correct. |
| 2. | Statement I is correct; Statement II is incorrect. |
| 3. | Statement I is incorrect; Statement II is correct. |
| 4. | Statement I is incorrect; Statement II is incorrect. |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
From NCERT
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Consider the given reaction:

Compound 'B' is:

Compound 'B' is:
| 1. | Crotonaldehyde | 2. | Acrolein |
| 3. | Mesityl oxide | 4. | Propanal |
Subtopic: Name Reaction |
51%
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.

The compound "A' is-
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Acid Derivatives - Preparation, Properties & Uses |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Which, of the following compounds, can give iodoform reaction?
| 1. | \(~~~~~~~~~~~~O\\ ~~~~~~~~~~~~||\\ HO-C-CH_3\) |
| 2. | \(~~~~~~~~~~~~~O\\ ~~~~~~~~~~~~~~||\\ H_2N-C-CH_3\) |
| 3. | \(~~~~~~~~~~~~~O\\ ~~~~~~~~~~~~~||\\ CH_3 -C - CH_3\) |
| 4. | All of the above |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.




