A person living in Shimla observed that cooking food without using a pressure cooker takes more time. The reason for this observation is-
1. Pressure increases
2. Temperature decreases
3. Pressure decreases
4. Temperature increases
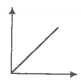
A plot of volume versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in the figure given below.
The correct order of pressure is -
Temperature (K)
1.
2.
3.
4.
As the temperature increases, average kinetic energy of molecules increases. With increase in temperature at constant volume, the pressure -
1. Increases
2. Decreases
3. Remains same
4. Becomes half
Match the graphs between the following variables with their names.
|
Graphs |
Names |
|
A. Pressure vs temperature graph at constant molar volume |
1. Isotherms |
|
B. Pressure vs |
2. Constant temperature Curve |
|
C. Volume vs temperature graph at constant pressure. |
3. Isochores |
|
4. Isobars |
Codes
| Options: | A | B | C |
| 1. | 3 | 1 | 4 |
| 2. | 1 | 2 | 3 |
| 3. | 5 | 4 | 3 |
| 4. | 4 | 5 | 3 |
Match the following gas law with the equation representing them.
|
A. Boyle’s law |
1. at constant T and p |
|
B. Charle’s law |
2. …constant T,V |
|
C. Dalton’s law |
3. constant |
|
D. Avogadro’s law |
4. at constant n and p |
|
5. at constant n and T |
Codes
| Options: | A | B | C | D |
| 1. | 5 | 4 | 2 | 1 |
| 2. | 3 | 4 | 2 | 1 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 3 | 2 |
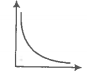
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
| Options: | A | B | C |
| 1. | 2 | 3 | 1 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 2 | 1 |
| 4. | 1 | 3 | 1 |