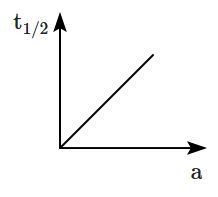
Consider the graph [between half-life \(\left(t_{1 / 2}\right)\) and initial concentration (a) of the reactant] for the reaction \(A \longrightarrow B,\)
The graph between \(-\dfrac{d[A]}{d t}\) and time will be:

The graph between \(-\dfrac{d[A]}{d t}\) and time will be:
| 1 | 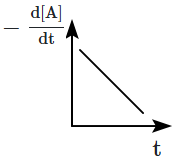 |
2 | 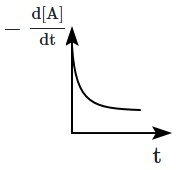 |
| 3 | 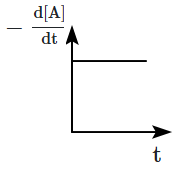 |
4 | 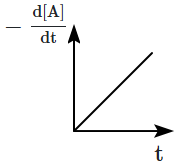 |
From NCERT
Subtopic: Order, Molecularity and Mechanism |
Please attempt this question first.
Please attempt this question first.
Please attempt this question first.
Launched MCQ Practice Books
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyConsider the reaction, 2A + B → Products.
When concentration of B alone was doubled, the half-life did not change. When the concentration of A alone was doubled, the rate increased by two times. The unit of rate constant for this reaction is:
1. L mol–1 s–1
2. no unit
3. mol L–1s–1
4. s–1
Subtopic: Order, Molecularity and Mechanism |
To view explanation, please take trial in the course below.
NEET 2026 - Target Batch - Vital
To view explanation, please take trial in the course below.
NEET 2026 - Target Batch - Vital
Please attempt this question first.
Launched MCQ Practice Books
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyWhich of the following statements is true regarding the nature of reaction order and reaction mechanisms?
| 1. | A second-order reaction is always a multistep reaction. |
| 2. | A zero-order reaction is a multistep reaction. |
| 3. | A first-order reaction is always a single-step reaction. |
| 4. | A zero-order reaction is a single-step reaction. |
From NCERT
Subtopic: Order, Molecularity and Mechanism |
Please attempt this question first.
Please attempt this question first.
Please attempt this question first.
Launched MCQ Practice Books
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & Biology