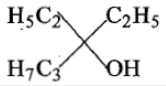
The compound which on reduction with gives two diffrent alcohols:
1.
2.
3.
4.
HCHO and HCOOH are distinguished by treating with:
1. Tollens reagent
2. NaHCO3
3. Fehling's Solution
4. Benedict Solution
Lacrymator or tear gas is:
1. C6H5COCl
2. C6H5OC6H5
3. C6H5COCH2Cl
4. C6H5COCH3
Formaldehyde can be distinguished from acetaldehyde by:
1. Fehling's solution
2. Schiff's reagent
3. Ammonia
4. Ammoniacal
The correct acidity order of the following is:


1. (III)>(IV)>(II)>(I)
2. (IV)>(III)>(I)>(II)
3. (III)>(II)>(I)>(IV)
4. (II)>(III)>(IV)>(I)
Acetone reacts with iodine (I2) to form iodoform in the presence of
1. CaCO3
2. NaOH
3. KOH
4. MgCO3
Generally Aldehydes behave as:
1. Oxidising agent
2. Reducing agent
3. Dehydration agent
4. Oxidizing as well as reducing agent
Compound (A) C5H10O forms a phenyl hydrazone and gives negative Tollen's and iodoform tests. Compound (A) on reduction gives n-pentane. Compound (A) is:
1. a primary alcohol
2. an aldehyde
3. a ketone
4. a secondary alcohol
RCOOH RCH2OH. This mode of reduction of an acid to alcohol can be affected only by:
1. Zn/HCl
2. Na-alcohol
3. aluminium isopropoxide and isopropyl alcohol
4. LiAlH4
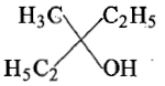
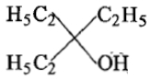
Ethyl ester P. the product P will be:
1. 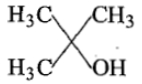

2. 

3. 

4.