The density of KCl is 1.9893 g cm-3 and the length of a side unit cell is 6.29082 Å as determined by X-ray diffraction. The value of Avogadro’s number
calculated from this data is:-
1. 6.017 x 1023
2. 6.023 x 1022
3. 7.03 x 1023
4. 6.01 x 1019
1. 6.017 x 1023
How many octahedral and tetrahedral holes are present per unit cell in a face centred cubic arrangement of atoms?
1. 8,4
2. 1,2
3. 4,8
4. 2 ,1
TiO2 is a well-known example of:
1. Triclinic system.
2. Tetragonal system.
3. Monoclinic system.
4. None of the above.
For a solid with the following structure, the co-ordination number of the point B is:
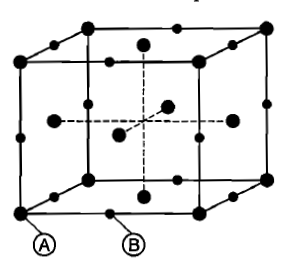
1. 3
2. 4
3. 5
4. 6
The elements of symmetry in a crystal are:-
1. Plane of symmetry
2. Axis of symmetry
3. Centre of symmetry
4. All of the above
Compute the percentage void space per unit volume of unit cell in zinc fluoride structure.
1. 15.03%
2. 22.18%
3. 18.23%
4. 25.07%
8 : 8 co-ordination of CsCl is found to change into 6 : 6 co-ordination on:
1. Applying pressure.
2. Increasing temperature.
3. Both 1 and 2.
4. None of these.
Frenkel defect is noticed in:
1. AgBr
2. ZnS
3. Agl
4. All of the above
Close packing is maximum in the crystal lattice of:
1. simple cubic
2. face centred
3. body centred
4. none of these
A spinel is an important class of oxides consisting of two types of metal ions with the oxide ions arranged in ccp layers. The normal spinel has 1/8th of the tetrahedral void occupied by one type of metal and one half of the octahedral voids occupied by another type of metal ions. Such a spinel is formed by Zn2+, Al3+ and O2- with Zn2+ in tetrahedral void. Give the simplest formula of the spinel.
1. ZnAl2O4
2. ZnAl2O3
3. ZnAlO
4. None of these






