Which of the following is more basic than aniline?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
Which one of the following has the shortest carbon-carbon bond length?
1. Benzene
2. Ethene
3. Ethyne
4. Ethane
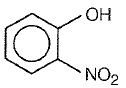
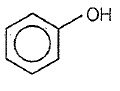
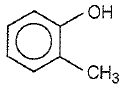
Which one of the following compounds is most acidic?
1. 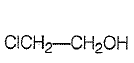
2. 
3. 
4. 
| 1. | 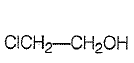 |
| 2. |   |
| 3. |   |
| 4. |   |
Which of the following represents the correct order of acidity in the given compounds?
1. FCOOH > COOH > BrCOOH > ClCOOH
2. BrCOOH > ClCOOH > FCOOH > COOH
3. FCOOH > ClCOOH > BrCOOH > COOH
4. COOH > BrCOOH > ClCOOH > FCOOH
Among the following, the strongest acid is:
| 1. | CH3COOH | 2. | CH2ClCH2COOH |
| 3. | CH2ClCOOH | 4. | CH3CH2COOH |
The distance between two adjacent carbon atoms is largest in
1. Benzene
2. Ethene
3. Butane
4. Ethyne
What is the correct order of increasing acidic strength among (I) p-methoxyphenol, (II) p-methylphenol, and (III) p-nitrophenol?
1. III < I < II
2. II < I < III
3. III < II < I
4. I < II < III
Which of the following represent the correct decreasing order of acidic strength of following?
(i) Methanoic acid
(ii) Ethanoic acid
(iii) Propanoic acid
(iv) Butanoic acid
1. (i)>(ii)>(iii)>(iv)
2. (ii)>(iii)>(iv)>(i)
3. (i)>(iv)>(iii)>(ii)
4. (iv)>(i)>(iii)>(ii)
The IUPAC name of the compound,
 is:
is:
1. 1, 3-Dibromo-3-methylbutane
2. 3-Methyl-1,2-bromobutane
3. 3-Methyl-1,3-bromopropane
4. None of the above
The shortest C-C bond distance is found in
1. Diamond
2. Ethane
3. Benzene
4. Acetylene






