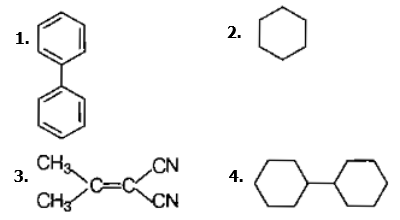
In which of the following molecules, all atoms are coplanar ?

Predict the correct order among the following.
(1) lone pair-lone pair>bond pair-bond pair>lone pair-bond pair
(2) bond pair-bond pair>lone pair-bond pair>lone pair-lone pair
(3) lone pair-bond pair>bond pair-bond pair>lone pair-lone pair
(4) lone pair-lone pair>lone pair-bond pair>bond pair-bond pair
The pair of electrons in the given carbanion, CH3C≡C-, is present in which of the following orbitals?
| 1. | sp3 | 2. | sp2 |
| 3. | sp | 4. | 2p |
The incorrect statement among the following regarding the molecules of CH4, NH3, and H2O is:
| 1. | The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4. |
| 2. | The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3. |
| 3. | The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3. |
| 4. | The H-C-H bond angle in CH4, the H-N-H bond angle in NH3, and the H-O-H bond angle in H2O are all greater than 90°. |
Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules?
1. Cl2 > Br2 > F2 > I2
2. Br2 > I2 > F2 > Cl2
3. F2 > Cl2 > Br2 > I2
4. I2 > Br2 > Cl2 > F2
Which of the following pairs of ions are isoelectronic and isostructural?
The correct bond order in the following species is
1.
2.
3.
4.
The total number of -bond electrons in the following structure is
1. 4
2. 8
3. 12
4. 16
In which of the following pairs, both the species are not isostructural?
1.
2. Diamond, silicon carbide
3.
4.







