The most suitable method of separation of 1:1 mixture of ortho and para-nitrophenols is
1. sublimation
2. chromatography
3. crystallisation
4. steam distillation
In pyrrole,
The electron density is maximum on
1. 2 and 3 2. 3 and 4
3. 2 and 4 4. 2 and 5
Which among the given molecules can exhibit tautomerisrn?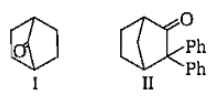
1. III Only
2. Both I and III
3. Both Iand II
4. Both II and III
The correct order of strengths of the carboxylic acids :-
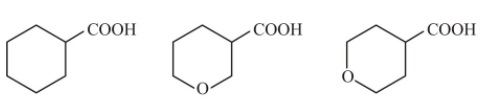
(a) I>II>III
(b) II>III>I
(c) III>II>I
(d) lI>I>IlI
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain |
| 3. | The staggered conformation of ethane is more stable than the eclipsed conformation because staggered conformation has no torsional strain |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has torsional strain |
In Duma’s method for estimation of nitrogen, 0.25g of an organic compound gave 40 mL
of nitrogen collected at 300 K temperature and 725 mm pressure. If the aqueous tension
at 300 K is 25 mm, the percentage of nitrogen in the compound is
1. 17.36
2. 18.20..
3. 16.76
4. 15.76
The compound that gives the most stable carbonium ion after C- Cl bond ionisation among the following is-
| 1. |  |
2. |  |
| 3. |  |
4. |  |
consider the following compounds
hyperconjugation occurs in
1. I only
2. II only
3. III only
4. I and III
Which of the following is the most correct electron displacement for a nucleophilic reaction to take place?
The enolic form of ethyl acetoacetate is given below. The number of sigma and pi bonds in the enolic form of ethyl acetoacetate are:
1. 18 sigma bonds and 2 pi-bonds
2. 16 sigma bonds and 1 pi-bond
3. 9 sigma bonds and 2 pi-bonds
4. 9 sigma bonds and 1 pi-bond










