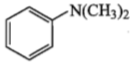
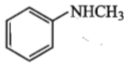
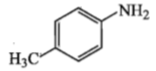
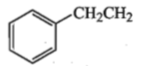
Amongst the compounds given, the one that would form a brillient coloured dye on treatment with NaNO2 in dil. HCl followed by addition to an alkaline solution of b-napthol is:
1. 
2. 
3. 
4. 




The presence of radical in solution can be detected by:
1. Fehling’s solution
2. Benedict’s solution
3. Schiff's reagent
4. Nessler’s reagent
Potassium thiocyanate solution reacts with ferric chloride to give:
(1) pink colour
(2) deep blue colour
(3) green colour
(4) blood-red colour
An organic compound X (mol. formula C6H5O2N) has 6 carbon atoms in a ring system, three double bonds and also a nitro group as substituent X is:
1. Homocyclic but not aromatic
2. Aromatic but not homocyclic
3. Homocyclic and aromatic
4. Heterocyclic
Choose the appropriate sequence to maximize the yield of 3-chloro-aniline from benzene.
1. Chlorination, nitration, reduction
2. Nitration, chlorination, reduction
3. Nitration, reduction, chlorination
4. Nitration, reduction, acylation, chlorination, hydrolysis
A reaction that can convert acetamide to methanamine is:
1. Carbylamine reaction
2. Hoffmann bromamide reaction
3. Stephens reaction
4. Gabriels phthalimide synthesis
Which one of the following nitro-compounds does not react with nitrous acid?
A given nitrogen-containing aromatic compound a reacts with Sn/HCl, followed by HNO2 to give an unsatable compound B.B, on treament with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is
The correct statement regarding the basicity of arylamines is
(1) Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring p-electron system.
(2) Arylamines are generally more basic than alkylamines because of aryl group.
(3) Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp-hybridized
(4) Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p-electron system.
The electrolytic reduction of nitrobenzene in a strongly acidic medium produces
1. p-Aminophenol
2. Azoxybenzene
3. Azobenzene
4. Aniline








