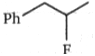
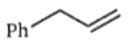
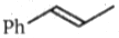
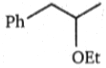
Two alkenes, X(91% yield) and Y(9% yield) are formed when the following compound is heated.

The structures of X and Y, respectively are:
1. 
2. 
3. 
4. 





In the dehydrohalogenation of 2-bromobutane; which conformation leads to the formation of cis-2-butene ?
(1)
(2)
(3)
(4)
CH3-CC-CH3
x and y mole consumed.
Value of x + y =
1. 5
2. 6
3. 7
4. 8
Br Br
l l
CH2-CH=CH-CH2→(A)
Zn(dust)
Above reaction is an example of 1,4-elimination.Predict the product.
(1) CH3-CH=C=CH2
(2) CH3-CC-CH3
(3) CH3-CH2-CCH
(4) H2C=CH-CH=CH2
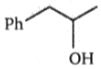
Which is the major product expected from the following SN2 reaction?
1. 
2. 
3. 
4. 
(a)
CH3-CH-CH3
l
Br
(b)
CH3-CH-Br
(c)
Compare rate of E2 reaction:-
(1)c>b>a
(2)a>b>c
(3)b>a>c
(4)c>a>b
Which is the major product of the following reaction?

1.
2.
3.
4.