Which of the following has a maximum 'Cl-O' bond order?
1.
2.
3.
4.
The correct statement regarding molecule is:
1. two bonds
2. molecules has 2 lone pair, 2 bonds and 2 bonds
3. two bonds
4. one and one bond
Which of the following species contains equal number of and bonds?
1.
2. XeO4
3. (CN)2
4. CH2(CN)2
What is the dominant intermolecular force on bond that must be overcome in converting
liquid CH3OH to a gas?
1. Hydrogen bonding
2. Dipole-dipole interaction
3. Covalent bonds
4. London dispersion force
The correct order of electronegativity of hybrid orbitals of carbon is:
(1) sp>sp2<sp3
(2) sp>sp2>sp3
(3) sp<sp2>sp3
(4) sp<sp2<sp3
The incorrect order of lattice energy is :
Which atom can have more than eight valence electrons when it is forming covalent bonds ?
1. H
2. N
3. F
4. Cl
How many sp and sp-hybridised carbon atoms are present respectively in the following compound ?
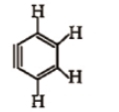
1. 4, 2
2. 6, 0
3. 3, 3
4. 5, 1
and both are covalent compounds but is polar whereas is non-polar. This is because :
1. Nitrogen atom is smaller than boron atom
2. N-F bond is more polar than B-F bond
3. NF is pyramidal whereas BF is planar triangular
4. BF is electron deficient whereas NF is not






