Compound P(C6H10) does not have any geometrical isomer. ON ozonolysis, two products R(C3H4O) and Q(C3H6O) are formed. R gives negative iodoform test while Q responds positively towards I2/NaOH solution. S, another isomer of P is an unsyumetrical alkene and on ozonolysis produces T(C6H10O2) which also gives a yellow precipitate with I2/NaOH solution and also gives positive test with Tollen's reagent. Which of the following does not represent any of the molecules amongst P,Q,R,S & T.
(1)

(2)

(3)

(4)





On halogenation, an alkane gives only one monohalogenated product. The alkane may be :
(1) 2-methyl butane
(2) 2, 2-dimethyl propane
(3) cyclopentane
(4) both (b) and (c)
Which of the following compounds can be best prepared by Wurtz-reaction ?
(1) Iso-butane
(2) n-butane
(3) n-pentane
(4) Iso-pentane

1. Three (3)
2. Four (4)
3. One (1)
4. Two (2)
\(\mathrm{CH}_3 \mathrm{Cl} \longrightarrow \mathrm{CH}_4\)
1. \(\mathrm{Zn} / \mathrm{H}^{+}\)
2. \(\mathrm{LiAlH}_4\)
3. \(\mathrm{Mg}\text{/(ether) then } \mathrm{H}_2 \mathrm{O}\)
4. All of the above

| 1. |  |
2. |  |
| 3. |  |
4. | Both (2) & (3) |
Determine the double bond equivalent (degree of unsaturation) of the compound 'A':
(A)
Given that compound 'A' undergoes hydrogenation with 1 mole of H₂ in the presence of Pt, yielding the depicted product, what is the double bond equivalent of 'A'?
1. 1
2. 2
3. 3
4. 4
Ethane is subjected to combustion process. During the combustion the hybrid state of carbon changes from:-
(1) sp2 to sp3
(2) sp3 to sp
(3) sp to sp3
(4) sp2 to sp2
The above reaction is an example of:-
1. isomerization
2. polymerization
3. cracking
4. de-hydrogenation
Consider the following compounds:
(I) 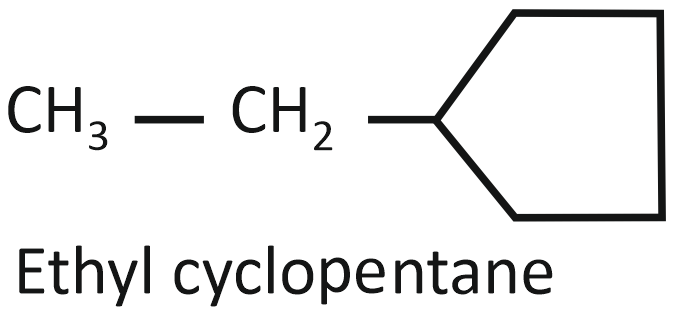
(II)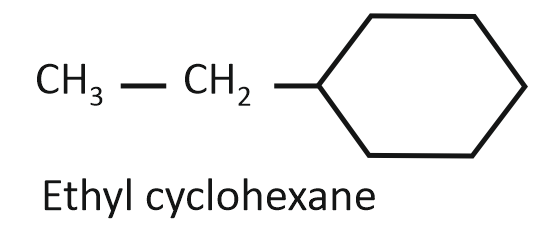
(III) 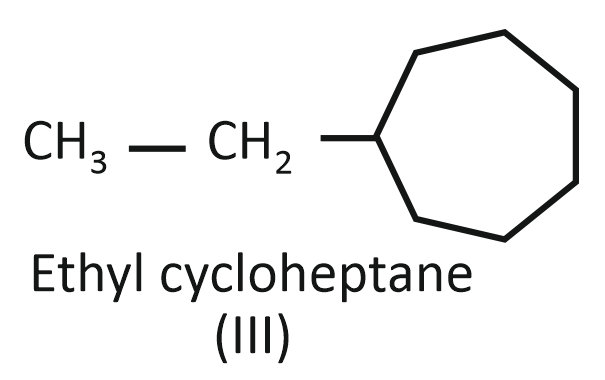
Arrange the compounds I, II, and III in decreasing order of their heats of combustion:
1. II>I>III
2. I>II>III
3. III>II>I
4. III>I>II







