Consider following compounds and decide as to which of the following statements are true ?

(1) (II) gives no reaction with Na metal, however, 1 mole of (IV) on reaction with Na metal will liberate 22.4 litres of H2 gas at STP
(2) (I) will give brisk effervescence on addition of NaHCO3 but will not bring any change in the colour of Br2 water
(3) (III) Iiberates H2 gas with Na metal, gives white precipitate with Tollen's reagent but does not respond towards lucas reagent or 2, 4–DNP test.
(4) (IV) gives turbidity with anhydrous ZnCI2

Which of the following carbocation will undergo rearrangement ?
1. 
2.
The colour of the solution that gets formed by mixing sodium nitroprusside to an alkaline solution of sulfide ions, is-
| 1. | Red | 2. | Blue |
| 3. | Brown | 4. | Purple |
In Kjeldahl's method, the nitrogen present is estimated as-
1. N2
2. NH3
3. NO2
4 None of the above
The Prussian blue colour obtained during the test of nitrogen by Lassaigne's test is due to the formation of-
| 1. | Fe4[Fe(CN)6]3 | 2. | Na3[Fe(CN)6] |
| 3. | Fe(CN)3 | 4. | Na4[Fe(CN)5NOS] |
A compound that does not give a positive test in Lassaigne’s test for nitrogen is:
1. Urea
2. Hydrazine
3. Azobenzene
4. Phenylhydrazine
The IUPAC name of the compound is:
1. 2-Methyl-6-oxohex-3-enamide
2. 6-Keto-2-methyl hexanamide
3. 2-Carbamoylhexanal
4. 2-Carbamoylex-3-enal
The correct IUPAC name of the given compound is:
1. Methyl 2-ethylbutanoate
2. 1-Methoxy-2-ethylbutanone
3. 3-Methoxycarbonylpentane
4. 1-methoxy-2-ethylbutanal
The IUPAC name of CH3-CH=CH-COOH is-
1. But-1-en-4-oic acid
2. 1-Hydroxybut-2-en-1-one
3. But-2-en-1-oic acid
4. But-2-en-4-oic acid
The IUPAC name of the below given compound is:
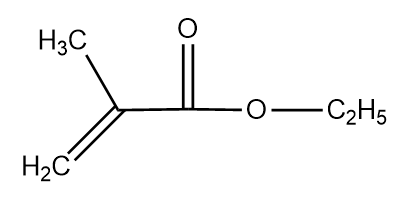
1. Ethyl 2-methylprop-2-enoate
2. Ethyl 2-methylprop-1-enoate
3. 1-Ethoxy 2-methylprop-2-enoate
4. 1-Ethoxy 2-methylprop-2-enal









