Cosider the following four elements, which are represented according to long form of periodic table.
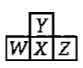
Here W, Y and Z are left, up and right elements with respect to the element 'X' and 'X' belongs to 16th group and 3rd period. Then according to given information the incorrect statement regarding given elements is:
1. Maximum electronegativity : Y
2. Maximum catenation property : X
3. Maximum electron affinity : Z
4. Y exhibits variable covalency
What is the atomic number of the element with the maximum number of unpaired 4p electrons?
1. 33
2. 26
3. 23
4. 15
The set representing the correct order of ionic radius is:
1.
2.
3.
4.
Calculate the amount of energy required to convert 110 mg of 'X' atom in gaseous state into X+ ion. (Atomic wt. for X=7 g/mol)
1. 10.4 kJ
2. 12.3 kJ
3. 11.3 kJ
4. 14.5 kJ
Which of the following orders of ionic radii is correctly represented?
| 1. | H–>H> | 2. | Na+>F–>O2– |
| 3. | F–>O2–>Na+ | 4. | N3–>Mg2+<Al3+ |
A pair among the following that have the same size is:
1. Fe3+, Ni2+
2. Zr4+, Ti4+
3. Zr4+ , Hf4+
4. Zn2+, Hf4+
The correct order of increasing electron gain enthalpy with a negative sign for the elements O, S, F, and Cl is :
1. Cl < F < S < O
2. O < S < F < Cl
3. F < S < O < Cl
4. S < O < Cl < F
With which of the following electronic configuration an atom has the lowest ionisation enthalpy?
(1) 1s22s22p5
(2) 1s22s22p3
(3) 1s22s22p63s1
(4) 1s22s22p6
Second ionization potential of Li, Be and B is in the order:
1. Li > Be > B
2. Li > B > Be
3. Be > Li > B
4. B > Be > Li
The order of ionisation potential between He+ ion and H-atom (both species are in gaseous state) is:
1. I.P. (He+) = I.P. (H)
2. I.P. (He+) < I.P. (H)
3. I.P. (He+) > I.P. (H)
4. cannot be compared






