BF3 is a planar and an electron deficient compound. Hybridization and number of electrons around the central atom, respectively are:
1.
sp2 and 6
2.
sp2 and 8
3.
sp3 and 4
4.
sp3 and 6
Match List-I with List-II
| List-I | List-II | ||
| (a) | (i) | Square pyramidal | |
| (b) | (ii) | Trigonal planar | |
| (c) | (iii) | Octahedral | |
| (d) | (iv) | Trigonal bipyramidal |
Choose the correct answer from the options given below:
| (a) | (b) | (c) | (d) | |
| 1. | (iii) | (i) | (iv) | (ii) |
| 2. | (iv) | (iii) | (ii) | (i) |
| 3. | (iv) | (iii) | (i) | (ii) |
| 4. | (ii) | (iii) | (iv) | (i) |
Which pair of ions among the following list do not constitute an iso-electronic pair?
1. Mn2+, Fe3+
2. Fe2+, Mn2+
3. O2–, F–
4. Na+, Mg2+
Which molecule among the following is non-polar?
| 1. | SbCl5 | 2. | NO2 |
| 3. | POCl3 | 4. | CH2O |
Which of the following correctly represents the Lewis dot structure of the CO molecule?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Which of the following represents the correct resonance structure of the carbonate ion ( \(CO_{3}^{2-}\))?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The following graph captures potential energy on the y-axis for hydrogen gas formation as a function of the internuclear distance on the x-axis:
The bond energy of H2 can be represented by:
| 1. | (c – a) | 2. | (b – a) |
| 3. | (c-a)/2 | 4. | (b-a)/2 |
The condition(s) required for the linear combination of atomic orbital is :
| 1. | Combining atomic orbitals must have the same or nearly the same energy. |
| 2. | Combining atomic orbitals must have proper orientations to ensure that the overlap is maximum. |
| 3. | Both 1 and 2 |
| 4. | None of the above |
The number of resonating structures exhibited by \(CO^{2-}_3\) ion are :
| 1. | 2 | 2. | 3 |
| 3. | 4 | 4. | 5 |
Consider the following structure.
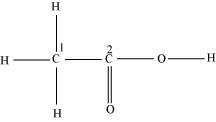
The hybridization of C1, and C2 carbon in the structure are -
1. = , =
2. = , =
3. = , =
4. None of the above







