A blacksmith fixes an iron ring on the rim of the wooden wheel of a horse cart. The diameter of the rim and the iron ring are 5.012 m and 5.00 m, respectively at 27 °C. To what temperature should the ring be heated so as to fit the rim of the wheel?
(Given: )
1.
2.
3.
4.
A sphere of 0.047 kg aluminium is placed for sufficient time in a vessel containing boiling water so that the sphere is at 100 °C. It is then immediately transferred to a 0.14 kg copper calorimeter containing 0.25 kg water at 20 °C. The temperature of water rises and attains a steady-state at 23 °C. The specific heat capacity of aluminium is:
(Given that: Specific heat capacity of copper calorimeter = and
the specific heat capacity of water )
1.
2.
3.
4.
When \(0.15\) kg of ice at \(0^\circ \text{C}\) is mixed with \(0.30\) kg of water at \(50^\circ \text{C}\) in a container, the resulting temperature is \(6.7^\circ \text{C}.\)
The heat of fusion of ice is: (\(S_{\text{water}}=4186\) J kg-1 K-1)
1. \( 3.43 \times 10^4\) Jkg-1
2. \( 3.34 \times 10^4\) Jkg-1
3. \( 3.34 \times 10^5\) Jkg-1
4. \(4.34 \times 10^5\) Jkg-1
The heat required to convert 3 kg of ice at –12 °C kept in a calorimeter to steam at 100 °C at atmospheric pressure is:
(Given, the specific heat capacity of ice = , the specific heat capacity of water = , the latent heat of fusion of ice =
and the latent heat of steam = .)
1.
2.
3.
4.
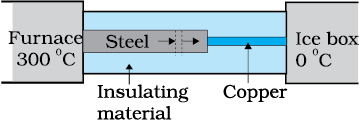
What is the temperature of the steel-copper junction in the steady-state of the system shown in the figure?
The length of the steel rod = 15.0 cm, length of the copper rod = 10.0 cm, temperature of the furnace = 300 °C, temperature of the other end = 0 °C. The area of the cross section of the steel rod is twice that of the copper rod.
(Thermal conductivity of steel = ; and of copper = ).
1.
2.
3.
4.
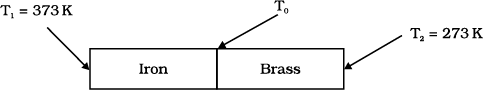
An iron bar and a brass bar are soldered end to end as shown in the figure. The free ends of the iron bar and brass bar are maintained at 373 K and 273 K respectively. The temperature of the junction of the two bars is:
1.
2.
3.
4.
A pan filled with hot food cools in 2 minutes from \(94^{\circ}\mathrm{C}\) to \(86^{\circ}\mathrm{C}\) when the room temperature is \(20^{\circ}\mathrm{C}\). How long will it take to cool from \(71^{\circ}\mathrm{C}\) to \(69^{\circ}\mathrm{C}\)?
| 1. | 50 sec | 2. | 52 sec |
| 3. | 42 sec | 4. | 48 sec |
An iron bar and a brass bar are soldered end to end as shown in the figure. The free ends of the iron bar and brass bar are maintained at 373 K and 273 K respectively and the The equivalent thermal conductivity of the compound bar is:

1.
2.
3.
4.
An iron bar and a brass bar are soldered end to end as shown in the figure. The free ends of the iron bar and brass bar are maintained at 373 K and 273 K respectively. The heat current through the compound bar is:

1.
2.
3.
4.
The given graph shows the variation of Fahrenheit temperature () vs Celsius temperature (). The correct relation between the two temperature scales that can be deduced from the graph below is



