An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one.
The IUPAC name of ‘A’ is :
1. 3-Ethylpent-2-ene
2. 3-Ethylpent-2- yne
3. 2-Ethylpent-3-ene
4. 3-Ethylpent-4-yne
The IUPAC name of ‘A’ is :
An alkene ‘A’ contains 3 C – Cσ bonds, 8 C – H σ bonds, and 1 C – C π bond,
‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. The IUPAC name of ‘A’ is:
| 1. | Prop-2-yne | 2. | But-2-ene |
| 3. | But-2-yne | 4. | Prop-2-ene |
Propanal and pentane-3-one are the products of ozonolysis of an alkene. The structural formula of the alkene is :
| 1. | 2. | ||
| 3. | 4. |  |
Benzene is extraordinarily stable, though it contains three double bonds, because of :
1. Delocalized protons
2. Delocalized neutrons
3. Resonance
4. None of the above.
Out of benzene, m-dinitrobenzene, and toluene, the one that will undergo nitration most easily is :
| 1. | Benzene | 2. | m–Dinitrobenzene |
| 3. | Toluene | 4. | None of the above |
Lewis acid(s) that can be used during the ethylation of benzene is/are:
| 1. |
|
2. |
|
| 3. |
|
4. | All of the above |
The IUPAC names of the following compounds are?
(a) CH3CH=C(CH3)2
(b) CH2=CH-C≡C-CH3
(c) 
(d)
(e)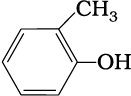
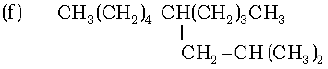
(g) CH3 – CH = CH – CH2 – CH = CH – CH – CH2 – CH = CH2
|
C2H5
1. 2-Methylbut-2-ene, Pen-1-ene-3-yne, 1, 3-Butadiene, 4-Phenyl but-1-ene , 2-Methyl phenol, 5-(2-Methylpropyl)-decane and 4-Ethyldeca-1, 5, 8-triene
2. 4-Phenyl but-1-ene, Pen-1-ene-3-yne, 1, 3-Butadiene, 2-Methylbut-2-ene, Pen-1-ene-3-yne, 2-Methyl phenol, 5-(2-Methylpropyl)-decane and 4-Ethyldeca-1, 5, 8-triene
3. 2-Methylbut-2-ene, 4-Phenyl but-1-ene, 1, 3-Butadiene, Pen-1-ene-3-yne, 2-Methyl phenol, 5-(2-Methylpropyl)-decane and 4-Ethyldeca-1, 5, 8-triene
4. 4-Ethyldeca-1, 5, 8-triene, Pen-1-ene-3-yne, 1, 3-Butadiene, 4-Phenyl but-1-ene, 2-Methyl phenol, 5-(2-Methylpropyl)-decane and 2-Methylbut-2-ene
The numbers of structural isomers of (with one double bond) and (with one triple bond) are:
1. \(\mathrm{C}_4 \mathrm{H}_8=3 ; \mathrm{C}_5 \mathrm{H}_8=3 \)
2. \(\mathrm{C}_4 \mathrm{H}_8=4 ; \mathrm{C}_5 \mathrm{H}_8=4 \)
3. \(\mathrm{C}_4 \mathrm{H}_8=3 ; \mathrm{C}_5 \mathrm{H}_8=4\)
4. \(\mathrm{C}_4 \mathrm{H}_8=4 ; \mathrm{C}_5 \mathrm{H}_8=3\)
Which of the following is the correct balanced chemical equation for the combustion of butane?
| 1. | \(\mathrm{2 {C}_4 {H}_{10({g})}+13 {O}_{2({g})} \rightarrow 8 {CO}_{2({g})}+10 {H}_2 {O}} + Heat \) |
| 2. | \(\mathrm{2 {C}_4 {H}_{10({g})}+13 {O}_{2({g})} \rightarrow 9 {CO}_{2({g})}+10 {H}_2 {O}+} Heat \) |
| 3. | \(\mathrm{2 {C}_4 {H}_{10({g})}+14 {O}_{2({g})} \rightarrow 8 {CO}_{({g})}+10 {H}_2 {O}}+ Heat \) |
| 4. | \( \mathrm{{C}_4 {H}_{10({g})}+13 {O}_{2({g})} \rightarrow 8 {CO}_{({g})}+10 {H}_2 {O}}+ Heat\) |
The dihedral angle of the least stable conformer of ethane is:
| 1. | 60° | 2. | 0° |
| 3. | 120° | 4. | 180° |






