Compare the stability of the two resonating structures given below and mark the correct option:

1. (I) is more stable than (II)
2. (II) is more stable than (I)
3. (I) and (II) both have the same stability
4. None of the above

| Assertion (A): | CCl4 doesn't give precipitate of AgCl on heating with AgNO3. |
| Reason (R): | CCl4 is a non-polar molecule. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
0.1688 g organic compound when analyzed by the Dumas method yields 31.7 mL of moist nitrogen measured at 14º C and 758 mm mercury pressure. The % of the nitrogen in the organic compound (Aqueous tension at 14 º C =12 mm) is:
1. 30.9%
2. 10%
3. 40%
4. 21.9 %
0.6 g of an organic compound was Kjeldahlised and NH3 evolved was absorbed into 50 mL of semi-normal solution of H2SO4. The residual acid solution was diluted with distilled water and the volume made up to 150 mL. 20 mL of this diluted solution required 35 mL of N/20 NaOH solution for complete neutralization.
1. 24.2%
2. 32.4%
3.27.6 %
4. 20.8%
In the Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of:
The latest technique for isolation, purification and separation of organic compounds is -
| 1. | Crystallisation | 2. | Distillation |
| 3. | Sublimation | 4. | Chromatography |
\(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{I}+\mathrm{KOH}(\mathrm{aq}) \rightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{KI} \)
1. Nucleophilic substitution reaction
2. Elimination reaction
3. Addition reaction
4. Electrophilic substitution reaction
IUPAC name of the given compound is :

1. 3-Phenylpropane
2. Phenyl-1-butane
3. 2-Benzylethane
4. Propyl benzene
The resonance hybrid structure will not exist for-
a. CH3OH
b. R - CONH2
c. CH3CH = CHCH2NH2
| 1. | a, and c | 2. | a, and b only |
| 3. | only a | 4. | b and c only |
The type of structural isomerism shown by given compounds is-
and 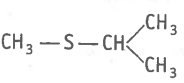
| 1. | Tautomerism | 2. | Positional isomerism |
| 3. | Functional isomerism | 4. | Ring Chain isomerism |






