If three elements A, B and C crystallized in cubic solid lattice with A atoms at corners, B atoms at cube centres and C atoms at the edges, the formula of the compound is
1. ABC
2. ABC3
3. AB3C
4. A3B2C3
In a cubic unit cell, seven of the eight corners are occupied by atom A and having of faces are occupied of B. The general formula of the substance having this type structure would be
1. A7B14
2. A14B7
3. A7B24
4. A9B24
In a cubic closed packed structure of mixed oxides, the lattice is made up of oxide ions, 20% of tetrahedral Voids are occupied by divalent A2+ ions and 50% of the octahedral voids by trivalent B3+. The formula of the oxide is?
1. A4B5O10
2. A2B5O5
3. A2BO
4. A4B5O8
A compound formed by elements A and B crystallizes in cubic structure where A atoms are at the corners or a cube and B atoms are at the face centre. The formula of the compound is :
1. AB
2. AB2
3. AB3
4. AB4
The radius of Ag+ ion is 126 pm while that of I- ion is 216 pm. The coordination number of Ag in AgI is
1. 8
2. 6
3. 4
4. 2
Which of the following crystals have 6:6 coordination?
1. MnO
2. NH4I
3. ZnS
4. none of these
TiO2 (rutile) shows 6:3 coordination. The solid having rutile like structure among the following is
1. KCl
2. SnO2
3. ZnS
4. none of these
A solid having unit cell made up of planes as shown in figure coordination number of 'X' is
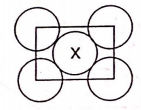
1. 12
2. 10
3. 6
4. 4
A compound contains two types of atoms: X and Y. It crystallizes in a cubic lattice with atoms X at the corners of the unit cell and atoms Y at the body centres.
The simples possible formula of this compound is:
1. XY
2. X2Y2
3. XY6
4. X8Y
The edge length of the unit cell of NaCl crystal lattice is 552 pm. If the ionic radius of sodium ion is 95 pm. What is the ionic radius of chloride ion?
1. 181 pm
2. 190 pm
3. 276 pm
3. 368 pm






