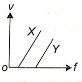
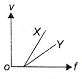
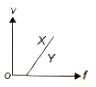
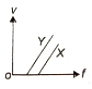
In a photoelectric experiment, electrons are ejected from metals X and Y by the light of intensity I and frequency f. The potential difference V required to stop the electrons is measured for various frequencies. If Y has a greater work function than X; which one of the following graphs best illustrates the expected results?
1. 
2. 
3. 
4. 





To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
An electron with the initial kinetic energy of 100 eV is accelerated through a potential difference of 50 V. Now the de-Broglie wavelength of electron becomes-
1. 1
2.
3.
4. 12.27

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
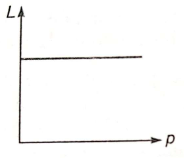
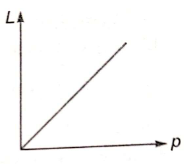
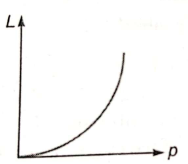
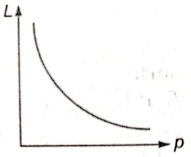
The de-Broglie wavelength L associated with an elementary particle of linear momentum p is best represented by the graph:
1. 
2. 
3. 
4. 

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
An electron of mass m, when accelerated through a potential difference, has de-Broglie wavelength .The de-Broglie wavelength associated with a proton of mass M accelerated through the same potential difference will be:
1.
2.
3.
4.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
An electron (mass m) with an initial velocity v= is in an electric E=. If , its de-Broglie wavelength at time t is given by
1.
2.
3.
4.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
What should be the velocity of an electron so that its momentum becomes equal to that of a photon of wavelength 5200 ?
1.
2.
3.
4.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
An electron and photon have the same wavelength. If E is the energy of photon and p is the momentum of the electron, then the magnitude of in SI unit is:
1.
2.
3.
4.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
The difference between kinetic energies of photoelectrons emitted from a surface by light of wavelength 2500 and 500 will be
1. 1.61 J
2. 2.47 J
3. 3.96 J
4.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
When a point source of light is 1 m away from a photoelectric cell, the photoelectric current is found to be I mA. If the same source is placed at 4 m from the same photoelectric cells, the photoelectric current (in mA) will be:
1.
2.
3.
4.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.
The work function of tungsten and sodium is 4.5 eV and 2.3 eV respectively. If the threshold wavelength for sodium is 5460 , the value of for tungsten is:
1. 2791
2. 3260
3. 1925
.4 1000

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 6 chapters you need to be enrolled in MasterClass Course.




